

The researchers obtained 3D imaging of the mouse liver with the nerves made clearly visible via a method that makes biological samples transparent using organic solvents (image: researchers’ archive).
In addition to providing a detailed description of the morphology of hepatic nerves, the study led by Brazilians showed that the increase in blood sugar was activated by a protein called CREB.
In addition to providing a detailed description of the morphology of hepatic nerves, the study led by Brazilians showed that the increase in blood sugar was activated by a protein called CREB.
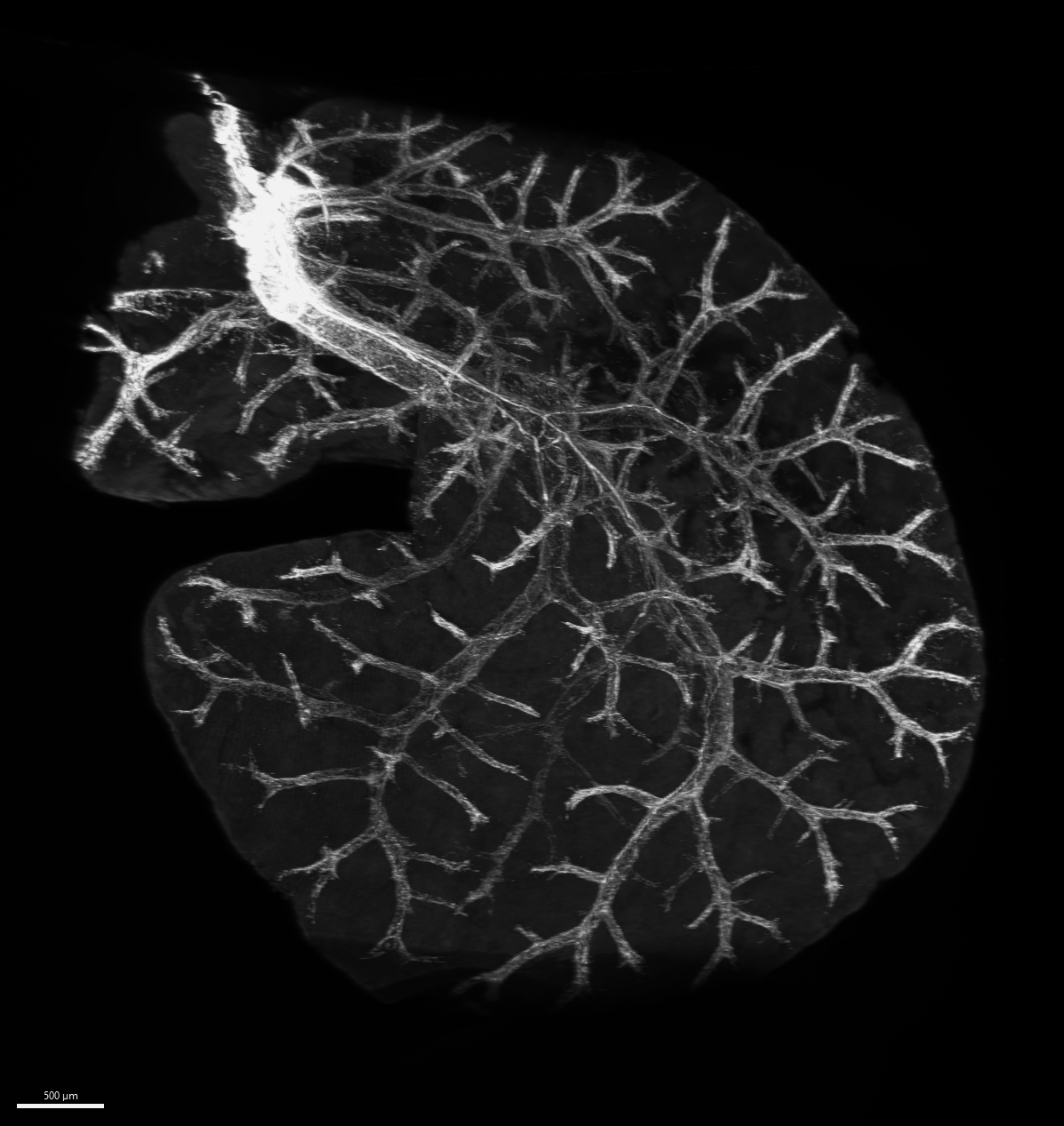
The researchers obtained 3D imaging of the mouse liver with the nerves made clearly visible via a method that makes biological samples transparent using organic solvents (image: researchers’ archive).
Por Luciana Constantino | Agência FAPESP – A study led by Brazilian researchers has produced a detailed description of the morphology of the nerves in the liver and how they control production of glucose when the organism is under stress. This process is known as hepatic gluconeogenesis. It is a key metabolic function of the liver that helps maintain a normal blood sugar level, especially when fasting and at times of high energy needs.
An article on the study, which entailed experiments with mice, is published in the journal Metabolism. According to the authors, the sympathetic nerves that stimulate the release of noradrenaline in the liver helped increase blood sugar by activating gluconeogenesis in response to cold. Specific molecules – the protein CREB and its coactivator CRTC2 – that have never been studied in depth were involved in this activation.
Noradrenaline (also called norepinephrine) is a neurotransmitter that plays a key role in rapid responses to stress or danger, raising heart rate, blood pressure and the release of glucose from energy reserves. Most studies in the scientific literature regarding the regulation of glucose synthesis by the liver focus on the action of hormones in the pancreas and adrenal glands.
A detailed analysis of these mechanisms is crucial to a better understanding of the disruptions of physiological processes that lead to metabolic diseases such as diabetes and obesity. The findings of the study therefore point to further opportunities for research on the same topic, especially situations involving alterations to the sympathetic nervous system, such as high blood pressure and hepatic steatosis (fatty liver disease).
“The originality of our study lies in showing that the central nervous system, via sympathetic nerves, can control CREB and activate de novo glucose production by the liver if extra energy is required. We describe the anatomy of the innervation in the liver using a methodology never used before in Brazil,” Luiz Carlos Navegantes, a professor in the Department of Physiology at the University of São Paulo’s Ribeirão Preto Medical School (FMRP-USP) and corresponding author of the article, told Agência FAPESP.
The researchers deployed a technique known as 3DISCO (three-dimensional imaging of solvent-cleared organs), a histology method that makes biological samples more transparent using organic solvents and produces a 3D image with the nerves clearly visible.
The study was supported by FAPESP via a Thematic Project, as well as master’s (19/26583-9 and 19/05900-6) and PhD scholarships awarded to biologist Henrique Jorge Novaes Morgan), first author of the article. Morgan won the Álvaro Osório de Almeida Prize awarded by the Brazilian Society of Physiology (SBFis) in 2021.
The other co-authors included researchers affiliated with the University of Oxford and Francis Crick Institute in the United Kingdom, and Marc Montminy at the Salk Institute for Biological Studies in the United States. The latter discovered CREB and elucidated its role in energy metabolism regulation, pointing to potential targets for drugs to combat insulin resistance, diabetes and obesity.
Methodology
The scientists used 3D immunoimaging to analyze the distribution of sympathetic nerves in the mouse liver, observing a dense innervation comprising thick primary nerve fibers with smaller branches that did not make direct contact with hepatocytes (liver cells).
To investigate the physiological role of this innervation, the mice were exposed to cold (4 °C) for up to six hours. Cold stress activated CREB/CRTC2 via calcium (Ca2+) signaling to assure the supply of glucose to muscles and keep the body warm.
“Continuing the groundbreaking research of Renato Hélios Migliorini, who founded our lab and showed in the 1980s that the nervous system was capable of activating gluconeogenesis, we set out to understand what happened at the molecular level. To this end, we analyzed mice from which we chemically or surgically removed this innervation in the liver, and transgenic mice without CRTC2. In these cases, gluconeogenesis did not occur in response to cold. In other words, mice without the nerves or the CREB coactivator were unable to produce glucose in response to stress. These findings added a novel component to the science of gluconeogenesis,” Navegantes said.
The article “Hepatic noradrenergic innervation acts via CREB/CRTC2 to activate gluconeogenesis during cold” is at: https://www.sciencedirect.com/science/article/pii/S0026049524001677.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





