

Study conducted by the FAPESP-funded Center for Research on Inflammatory Diseases (CRID) shows that drug currently used to treat cystic fibrosis can help prevent complications from infection by SARS-CoV-2 (image: confocal microscope image of NET being released by neutrophil / CRID-USP)
Study conducted by the FAPESP-funded Center for Research on Inflammatory Diseases (CRID) shows that drug currently used to treat cystic fibrosis can help prevent complications from infection by SARS-CoV-2.
Study conducted by the FAPESP-funded Center for Research on Inflammatory Diseases (CRID) shows that drug currently used to treat cystic fibrosis can help prevent complications from infection by SARS-CoV-2.
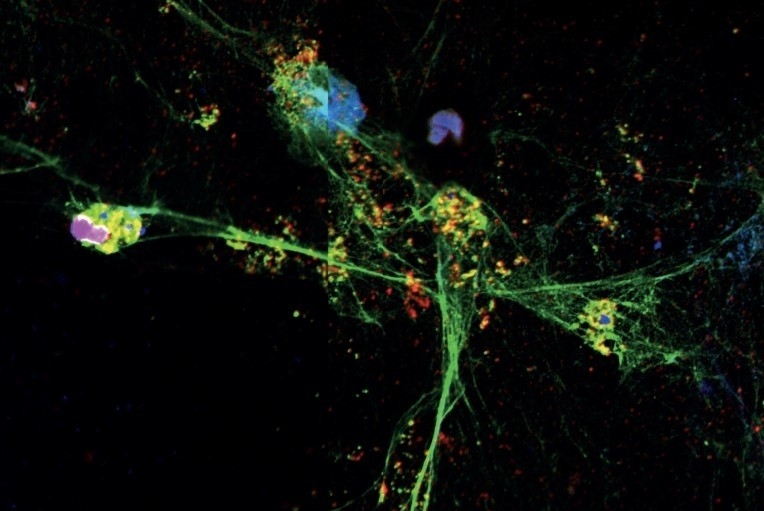
Study conducted by the FAPESP-funded Center for Research on Inflammatory Diseases (CRID) shows that drug currently used to treat cystic fibrosis can help prevent complications from infection by SARS-CoV-2 (image: confocal microscope image of NET being released by neutrophil / CRID-USP)
By Karina Toledo | Agência FAPESP – Severe COVID-19 patients develop a runaway inflammatory response that damages the organism and closely resembles what happens in sepsis. Experiments conducted at the University of São Paulo (USP) in Brazil by researchers affiliated with the Center for Research on Inflammatory Diseases (CRID) have shown that the same immune mechanism is involved in the body’s response to both infections.
Reported in an article posted to the platform medRxiv and not yet peer-reviewed, the discovery paves the way for novel approaches to the treatment of infection by SARS-CoV-2, including the repurposing of a drug currently used to treat cystic fibrosis whose active principle is an enzyme called DNase.
“In vitro tests using blood plasma from patients admitted to hospital with severe COVID-19 showed that DNase can deactivate the inflammatory immune mechanism that damages vital organs. We’re now working with the pharmaceutical company that makes the drug to see if it will be possible to conduct a clinical trial,” Fernando de Queiroz Cunha told Agência FAPESP. Cunha is principal investigator for CRID, a Research, Innovation and Dissemination Center (RIDC) funded by FAPESP and hosted by USP’s Ribeirão Preto Medical School (FMRP).
Sepsis involves systemic inflammation triggered by a localized infection that spirals out of control. The immune response unleashed to combat the pathogen is so strong that it ends up damaging the organism itself. The most severe form of sepsis can lead to lesions that impair the functioning of vital organs.
“COVID-19 is initially different because it’s a viral infection, but at some point it becomes very similar to sepsis. The inflammatory mediators are the same, and we observed participation by NETs [neutrophil extracellular traps] in both cases,” Cunha said.
Neutrophils are white blood cells that form the front line of the immune system, phagocytizing (killing) bacteria, fungi and viruses. NETs are structures composed of DNA and granular proteins, which rapidly trap and kill pathogens. In some situations, for poorly understood reasons, the process of NET formation leads to the activation of PAD-4, an intracellular enzyme that migrates to the neutrophil’s nucleus and increases the permeability of the nuclear membrane. The enzyme plays a key role in decondensation of the genetic material contained in the nucleus, so that NETs can be formed and released into the extracellular medium to trap and kill potential invaders.
This immune mechanism has been observed in patients with autoimmune diseases and subjects infected by chikungunya, an arbovirus that also produces lesions in tissue. “The problem is that NETs aren’t just toxic to pathogens – they also damage human cells. The good news is that according to our studies the enzyme DNase can cut disrupt the NETs released by neutrophils and avoid tissue damage,” Cunha said.
Preclinical trials
Recent research has shown that infection by SARS-CoV-2 can damage the lungs, heart, kidneys, nerves and skin. To confirm their suspicion that NETs could be involved in attacks on tissue, the CRID researchers analyzed blood plasma samples from 32 patients admitted to hospital with COVID-19 and compared them with plasma samples from healthy subjects.
In the case of 17 participants in the study who were treated in intensive care units (ICUs) with mechanical ventilation, it was also possible to collect samples of airway secretion discharge.
FMRP basic and clinical area faculty collaborated. The clinical group is led by Professor Paulo Louzada Junior. Several PhD candidates and postdoctoral fellows also participated in the study, including Flavio Protássio Veras, first author of the article.
“We found the plasma of patients hospitalized with COVID-19 to be full of NETs. Indeed, the quantity of these neutrophil traps in their airway secretion was ten times higher than in the controls. This suggests neutrophils are producing NETs throughout the organism but production is concentrated in the lungs,” Cunha said.
The finding was confirmed by an analysis of lung tissue samples from people who died as a result of COVID-19, thanks to a partnership with a group led by Paulo Saldiva, a professor in the University of São Paulo’s Medical School (FM-USP).
By means of immunofluorescence, a method that uses antibodies chemically labeled with fluorescent dyes to visualize molecules, the CRID researchers showed that NETs were present in large quantities in lung inflammation foci.
“In one of the experiments we isolated neutrophils in blood from healthy subjects and incubated them with SARS-CoV-2. As soon as they were infected, the defense cells started producing NETs,” Cunha said.
The infected neutrophils were then put in contact with cultured epithelial cells taken from human lung tissue. The epithelial cells died after a few hours of interaction with the infected neutrophils. The same lethal effect occurred when the neutrophils isolated from COVID-19 patients were placed in the pulmonary epithelial cell culture.
“We were able to avoid the death of epithelial cells by treating the infected neutrophils with DNase before placing them in the lung cell culture medium,” Cunha said.
Besides DNase, which disrupted the NETs after their release by defense cells, the researchers also tested a compound that inhibited the action of PAD-4 and thereby prevented the formation of NETs. In this case as well the treatment averted the death of pulmonary epithelial cells, although the substance tested has not yet been approved for human use.
“The article presents evidence that the DNase currently prescribed for cystic fibrosis can be tested as a treatment for severe COVID-19,” Cunha said. “However, the drug has to be inhaled and will be difficult to deliver to intubated patients, so perhaps it should be used to treat patients in an earlier stage of the disease, when blood oxygenation levels start to fall.”
Clinical trials are still necessary to find the ideal dose and the optimal timing for administration of the drug, he stressed.
In partnership with researchers affiliated with the Bioscience National Laboratory (LNBio) at the Brazilian Center for Research in Energy and Materials (CNPEM) in Campinas, in the state of São Paulo, the CRID group are also working on the development of a new compound to inhibit PAD-4 and disrupt the production of NETs. The results of the study, initially designed to find a treatment for sepsis, will be published soon.
The article “SARS-CoV-2 triggered neutrophil extracellular traps (NETs) mediate COVID-19 pathology” can be downloaded at: www.medrxiv.org/content/10.1101/2020.06.08.20125823v1.full.pdf.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





