

Discovery by Brazilian scientists paves the way for the study of more potent molecules capable of directly destroying parasites underlying elephantiasis and cutaneous leishmaniasis, with fewer adverse side-effects (3D structure of protein deoxyhypusine synthase from Brugia malayi; image: CQMED)
Discovery by Brazilian scientists paves the way for the study of more potent molecules capable of directly destroying parasites underlying elephantiasis and cutaneous leishmaniasis, with fewer adverse side-effects.
Discovery by Brazilian scientists paves the way for the study of more potent molecules capable of directly destroying parasites underlying elephantiasis and cutaneous leishmaniasis, with fewer adverse side-effects.
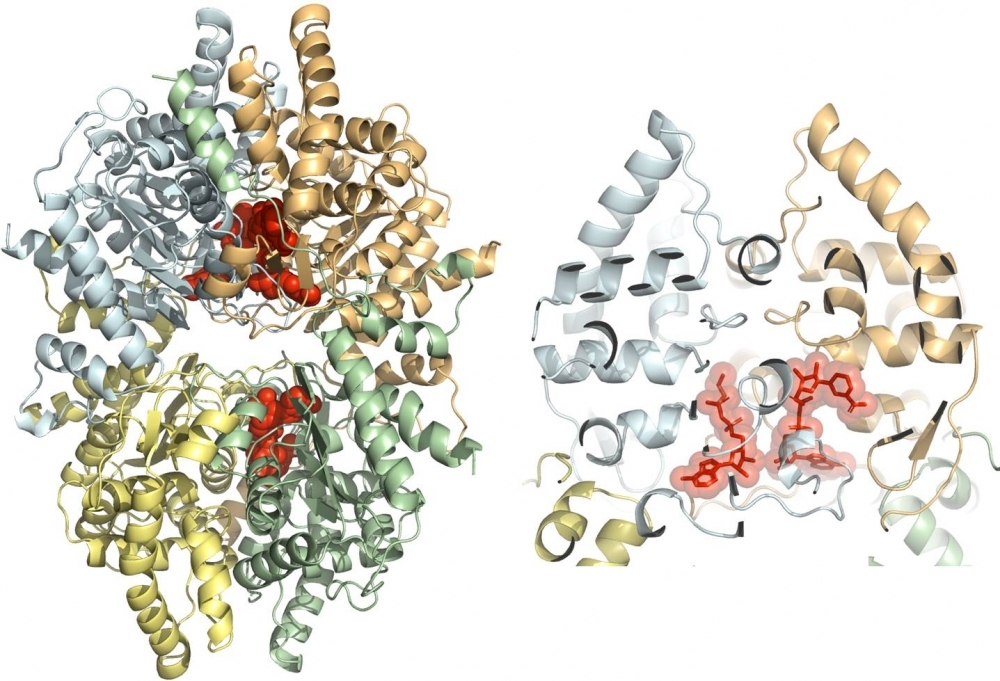
Discovery by Brazilian scientists paves the way for the study of more potent molecules capable of directly destroying parasites underlying elephantiasis and cutaneous leishmaniasis, with fewer adverse side-effects (3D structure of protein deoxyhypusine synthase from Brugia malayi; image: CQMED)
By Luciana Constantino | Agência FAPESP – Brazilian researchers have managed to decipher the structure of a protein found in parasites that cause neglected tropical diseases, paving the way to the development of novel medications. Thanks to the discovery it will be possible to seek more potent molecules capable of destroying the pathogens directly, with fewer adverse side-effects for patients.
The study detailed the structural characteristics of the protein deoxyhypusine synthase (DHS), found in Brugia malayi, one of the mosquito-borne parasites that cause elephantiasis, and in Leishmania major, the protozoan that causes cutaneous leishmaniasis.
Elephantiasis, also known as lymphatic filariasis, is an infection of the lymph system that can lead to swelling of the legs, arms, and genitalia. It may also harden and thicken the skin, limiting movement, and hindering normal activities.
Cutaneous leishmaniasis produces skin lesions weeks or months after the insect bite that transfers the parasite. Lesions may persist for years and leave scars similar to those caused by burns. Over 300,000 cases were notified in Brazil between 2003 and 2018, according to data from the Ministry of Health.
The study is reported in an article published in PLOS Neglected Tropical Diseases. The first authors are Suélen Silva and Angélica Klippel, PhD candidates at São Paulo State University (UNESP) in Araraquara. Silva is in the Biotechnology Program, and Klippel is in Bioscience and Biotech Applied to Pharmacy, with a scholarship from FAPESP, supervised by Cleslei Zanelli.
The research is conducted under the aegis of the National Institute of Science and Technology (INCT) for Open-Access Medicinal Chemistry hosted by the University of Campinas’s Center for Medicinal Chemistry (CQMED-UNICAMP). It receives funding from FAPESP, and federally from the National Council for Scientific and Technological Development (CNPq) at the Ministry of Science and Technology and CAPES, the Ministry of Education’s Coordination for the Improvement of Higher Education Personel.
The group achieved two important major advances with regard to DHS: standardization of a yeast-based platform to study the enzyme for Leishmania major, and three-dimensional assembly of the molecule found in the elephantiasis protozoan.
The identification of this novel target will now be followed by more research to develop or find molecules that inhibit DHS-mediated biochemical processes and stop the disease from progressing. If specific inhibitors are identified as the basis for drug development, it will be possible to reduce or completely avoid the adverse side-effects of current treatments, such as fever, nausea, and insomnia. In the case of elephantiasis, some medications are not even capable of killing adult worms.
“At CQMED we try to elucidate proteins that haven’t been studied in depth and to determine their crystal structure. The study revealed the structure of DHS in these parasites for the first time. We began with these two parasites [Brugia and Leishmania], but we want to go on to investigate four more organisms including Plasmodium, which causes malaria,” Katlin Massirer, principal investigator of the INCT, told Agência FAPESP. Massirer is affiliated with the University of Campinas’s Center for Molecular Biology and Genetic Engineering (CBMEG-UNICAMP).
Crystallography is an essential tool in research on the three-dimensional structure of proteins. Scientists use the technique to analyze the positions of the proteins’ atoms and their interactions, to understand how they act in the human organism, and to investigate how a prospective drug will have to bind to a given protein. Analyzing crystal structure also helps understand how each drug works so as to enhance its efficacy.
The study included in vitro synthesis of the active enzyme, the form in which DHS acts on the parasite, and assays to test its activity while screening the molecules that could become novel medications.
“The structure of the protein serves as a basis on which to identify the differences and try to find an inhibitor that will fit into the parasite’s enzyme without affecting the similar protein found in humans. It’s a big challenge,” Klippel said.
Progress
According to Massirer, it takes between 12 and 15 years on average to research, develop and bring novel medications to market, but in the case of neglected tropical diseases (NTDs) lead times can be even longer because so little research is done on these health problems. “You could say that the stage we’re at is equivalent to two years of this process,” she said.
NTDs are estimated to affect some 1.5 billion people in more than 150 countries, mainly in the world’s poorest regions. Besides leishmaniasis and elephantiasis, the list of NTDs published by the World Health Organization (WHO) includes Chagas disease, schistosomiasis, dengue fever, and chikungunya.
More than 350 research institutions, civil society organizations, companies, and governments of countries around the world signed up to celebrate the first-ever World NTD Day on January 30, 2020. The date was chosen because it was the anniversary of the 2012 London Declaration on NTDs, which redoubled global efforts to eradicate NTDs. Pharmaceutical companies, donors, endemic countries, and NGOs committed to control, eliminate or eradicate ten diseases by 2020 and improve the lives of over a billion people. Not all the goals have been met, however.
Scant interest in NTDs was one of the reasons Klippel chose to work on the problem. “For my PhD research I wanted to leverage my training as a pharmacist and to focus on a neglected area, to participate in something important but not highly valued,” she said. “Every stage is a victory. We’ve built a tool, and now we must take the next step.”
At CQMED, Massirer explained, the next step will consist of acquiring DHS from other species and at the same time searching repositories for molecules at home and abroad. The research has been proceeding more slowly because of the COVID-19 pandemic. “Our center lets groups from anywhere in Brazil propose innovative projects for collaborative research by the network,” she said.
The institute’s mission includes fostering partnerships among researchers at different centers to investigate little-studied proteins and increase the impact of discoveries.
CQMED is a unit of the Brazilian Agency for Industrial Research and Innovation (EMBRAPII). It was created with support from FAPESP through its Research Partnership for Technological Innovation Program (PITE), in cooperation with the Structural Genomics Consortium (SGC), a collaborative network of academics, pharmaceutical companies, and funding agencies working together to accelerate drug development.
The article “Structural features and development of an assay platform for the parasite target deoxyhypusine synthase from Brugia malayi and Leishmania major” can be read at: journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0008762.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





