
Labile iron prevents oxidation when it interacts with peroxynitrite, shows a study conducted at the Center for Research on Redox Processes in Biomedicine. The discovery has an impact on various biological processes (illustration: Redoxome RIDC)
Labile iron prevents oxidation when it interacts with peroxynitrite, shows a study conducted at the Center for Research on Redox Processes in Biomedicine. The discovery has an impact on various biological processes.
Labile iron prevents oxidation when it interacts with peroxynitrite, shows a study conducted at the Center for Research on Redox Processes in Biomedicine. The discovery has an impact on various biological processes.

Labile iron prevents oxidation when it interacts with peroxynitrite, shows a study conducted at the Center for Research on Redox Processes in Biomedicine. The discovery has an impact on various biological processes (illustration: Redoxome RIDC)
By Maria Fernanda Ziegler | Agência FAPESP – Scientists have long considered the labile iron pool a solely pro-oxidative cellular iron source. Labile iron is generally associated with the production of the highly reactive hydroxyl radical, which forms through a redox reaction with hydrogen peroxide. The hydroxyl radical is the most reactive oxygen species and is known to be capable of damaging important macromolecules.
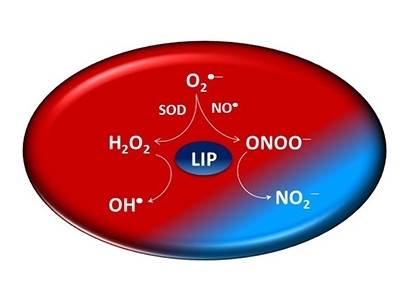
A study conducted at the Center for Research on Redox Processes in Biomedicine (Redoxome), one of the Research, Innovation and Dissemination Centers (RIDCs) funded by FAPESP, has shown that labile iron also displays anti-oxidative activity when it reacts with peroxynitrite.
In addition to being a powerful oxidant, peroxynitrite gives rise to other reactive free radicals. In physiological terms, this means labile iron also combats other oxidative substances.
“Labile iron has hitherto been considered an oxidative agent because of its interaction with hydrogen peroxide and its capacity to produce free radicals that damage macromolecules. However, we discovered that when labile iron interacts with another important peroxide, peroxynitrite, it acts in the opposite direction by preventing oxidation. Given that everything on the subject of labile iron in the scientific literature relates to its oxidative action, we believe this discovery marks an important paradigm shift,” said José Carlos Toledo Junior, a professor in the Chemistry Department of the University of São Paulo’s Ribeirão Preto School of Philosophy, Science and Letters (FFCLRP-USP) in Brazil and one of the authors of an article on the results of the study published in the Journal of Biological Chemistry.
The discovery by the Redoxome team contributes to a broader understanding of key cellular processes, since all cells contain labile iron and control its levels.
According to Toledo, peroxynitrite is a biologically relevant oxidant because it is produced by the recombination of two free radicals, nitric oxide (NO) and superoxide, to which most cells are normally exposed.
“Every human cell has labile iron, and every cell is exposed to NO and superoxide at some time. Peroxynitrite is produced in cells mainly in cases of infection or inflammation, where immune system cells produce both NO and superoxide to combat microorganisms and thus produce oxidants,” Toledo told Agência FAPESP.
“Knowing about the antioxidative activity of labile iron changes some ideas and may alter our understanding of biological processes that involve nitric oxide, peroxynitrite and labile iron.”
In this study, the researchers investigated the interaction between labile iron and peroxynitrite in laboratory-grown murine macrophages. The group also performed experiments using a cell-free system with equivalent conditions and the same indicators.
They used chelators (small molecules that bind very tightly to metal ions) to react with labile iron, change its properties and remove it from macrophages to see whether oxidation increased, using fluorescence spectroscopy to measure the oxidation.
“Everything we did in the experiment pointed to a different phenomenon from that described in the literature,” Toledo said. “The rise in oxidation when the iron was removed led us to suspect that labile iron might be an antioxidant. We pursued this hypothesis and showed it was true.”
Although labile iron’s interaction with peroxynitrite had never been considered, it was not exactly a surprise because metals are among peroxnyitrite’s preferred targets. “The reaction between labile iron and peroxynitrite proved kinetically competitive. What’s interesting is that the product of this reaction is not oxidative,” Toledo said.
“The discovery permits the development of a new research line relating to the antioxidative activity of labile iron. We plan to perform fundamental studies on reaction speed to see if this class of iron also reacts with peroxynitrite-derived radicals and, bearing in mind that cells contained differing amounts of labile iron, to see if its antioxidative activity toward nitric oxide is proportional to its amount.”
The article “The labile iron pool attenuates peroxynitrite-dependent damage and can no longer be considered solely a pro-oxidative cellular iron source” (doi: 10.4172/2165-7025.1000394) by Fernando Cruvinel Damasceno, André Luis Condeles, Angélica Kodama Bueno Lopes, Rômulo Rodrigues Facci, Edlaine Linares, Daniela Ramos Truzzi, Ohara Augusto and Jose Carlos Toledo Jr. can be retrieved from www.jbc.org/content/early/2018/04/16/jbc.RA117.000883.abstract?sid=afafc375-5a48-4a74-85c4-f6c96382e05e.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





