
Partnership between researchers in Brazil and Portugal permits application of sustainable process on industrial scale for purification of PEGylated proteins (image: release)
Partnership between researchers in Brazil and Portugal permits application of sustainable process on industrial scale for purification of PEGylated proteins.
Partnership between researchers in Brazil and Portugal permits application of sustainable process on industrial scale for purification of PEGylated proteins.

Partnership between researchers in Brazil and Portugal permits application of sustainable process on industrial scale for purification of PEGylated proteins (image: release)
By Maria Fernanda Ziegler | Agência FAPESP – Researchers in the Department of Biochemical & Pharmaceutical Technology (FBT) at the University of São Paulo’s Pharmaceutical Science School (FCF-USP) in Brazil, in partnership with colleagues in the University of Aveiro’s Chemistry Department in Portugal, have developed an alternative method of purifying PEGylated proteins, a class of biotherapeutic medication.
The study was supported by FAPESP and its results were published in a paper featured on the cover of the journal Green Chemistry.
In the paper, the research team describes an innovative and sustainable method of purifying modified proteins based on aqueous biphasic systems (ABSs). These purification platforms use water as a solvent and are highly protein-compatible, cost-effective, and easily scalable.
PEG stands for polyethylene glycol. PEGylation consists of the covalent attachment of polyethylene glycol chains to proteins and is now a well-established technology for targeted delivery of drugs and “biobetters”, new, improved versions of already approved biological drugs. Examples of PEGylated proteins include biotherapeutics administered to treat such diseases as severe combined immunodeficiency (SCID) or acute lymphoblastic leukemia (ALL).
The paper proposes PEGylation of a protein that can be used as a biosensor, describing a method for improving the stability of the protein by means of PEGylation and enhancing its effectiveness in this application.
“PEGylation has become a very important process in the pharmaceutical industry for the enhancement of biopharmaceuticals, and the number of biological drugs that are PEGylated proteins has increased considerably. However, PEGylation presents challenges, purification perhaps being the most daunting. This process corresponds to about 80% of the total cost of the drug,” said Carlota Rangel Yagui, one of the authors of the paper and principal investigator for the project supported by FAPESP.
Industrially, PEGylated proteins are purified by chromatography. There are drawbacks to this method, such as its high cost and the fact that non-PEGylated proteins cannot be recycled in a new PEGylation process, which would make the method more sustainable and profitable.
“We succeeded in performing purification of the molecules in three stages, making the process more sustainable and cost-effective than chromatography,” Yagui said. “ABSs, consisting only of water and two other substances such as a polymer or a salt, are already used to purify other substances and prove highly efficient for PEGylated proteins.”
Cytochrome c application as biosensor
The study was performed with cytochrome c, a protein that catalyzes oxidative processes and can be deployed as an analytical device or biosensor to detect alterations in samples. However, the researchers envisage adaptation of this alternative purification process for use with other PEGylated proteins.
“Biosensors are molecules of industrial interest, not only for the pharmaceutical industry but also for the food industry and agroindustry,” Yagui said.
The ABS-purified proteins were fractionated into different forms of PEGylated cytochrome c and separated from the proteins that did not react.
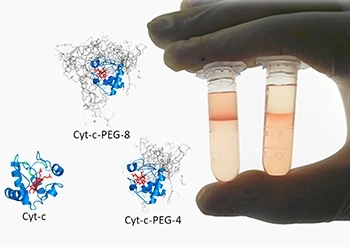
“We succeeded in PEGylating cytochrome c with four PEG molecules and also with eight. Nothing in the literature had ever described simultaneous separation of specific PEGylated forms and recycling of the native protein for a new PEGylation process,” said lead author João Santos, who is researching for two PhDs, one at USP and the other at the University of Aveiro.
The discovery of a sustainable alternative technique to purify PEGylated proteins led to the opening up of other lines of investigation by the group of researchers, especially scaling up the alternative technique and using PEGylated cytochrome c as a biosensor in electrochemical trials.
“In our article, we proposed cytochrome c as a biosensor, and we now want to find out whether PEGylation extends its working life,” Yagui said. Enzymatic biosensors are short-lived owing to denaturation by variations in temperature and pH, as well as exposure to the material used in the electrodes.
Santos has completed the study on industrial-scale ABS purification of PEGylated cytochrome c using centrifugal partition chromatography (CPC). A paper describing its results will be published later this year.
The Green Chemistry article “Multistep purification of cytochrome c PEGylated forms using polymer-based aqueous biphasic systems” (doi: 10.1111/1462-2920.14085) by João H. P. M. Santos, Gustavo Carretero, João A. P. Coutinho, Carlota O. Rangel-Yagui and Sónia P. M. Ventura can be retrieved from: pubs.rsc.org/en/content/articlelanding/2017/gc/c7gc02600e#!divAbstract.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





