

Nucleus of a liver cell from a healthy mouse (left, stained blue); liver from a mouse infected by murine hepatitis virus with arrows pointing to expression of the protein TMEM176D (right, stained red) (image: Maite D. Veja et al.)
A type of treatment known as immune checkpoint blockade proved beneficial in cells from COVID-19 patients in intensive care and in mice infected by a betacoronavirus similar to SARS-CoV-2. The study is published in Science Advances.
A type of treatment known as immune checkpoint blockade proved beneficial in cells from COVID-19 patients in intensive care and in mice infected by a betacoronavirus similar to SARS-CoV-2. The study is published in Science Advances.
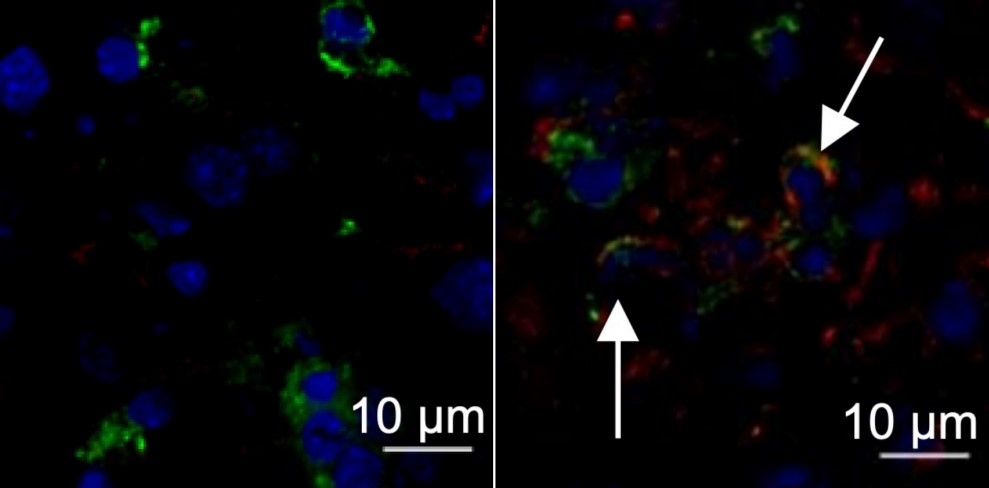
Nucleus of a liver cell from a healthy mouse (left, stained blue); liver from a mouse infected by murine hepatitis virus with arrows pointing to expression of the protein TMEM176D (right, stained red) (image: Maite D. Veja et al.)
By André Julião | Agência FAPESP – An article published in Science Advances suggests that a type of cancer treatment known as immune checkpoint blockade may be beneficial in certain cases of severe COVID-19. The creators of this therapy, which can successfully activate the immune system to fight cancer, won the 2018 Nobel Prize in Physiology or Medicine.
The findings reported by the authors were based on experiments involving cells from patients treated in intensive care units (ICUs) after being infected by SARS-CoV-2, and mice infected by MHV-A59 (murine hepatitis virus A59), another betacoronavirus.
“PD-1 blockade is one of the known immune checkpoint therapies and one of the therapies we analyzed in the study. It tells T lymphocytes [a type of white blood cell] to stop responding to infection after a time so that the response is not excessive. In cases of cancer, sepsis and severe COVID-19, however, PD-1 makes T cells stop functioning even before the disease has been resolved and must therefore be blocked,” said Pedro Moraes-Vieira, one of the leaders of the study. Moraes-Vieira is a professor at the State University of Campinas’s Institute of Biology (IB-UNICAMP) in São Paulo, Brazil, and supported by FAPESP.
Another co-author is Gustavo Gastão Davanzo, a PhD candidate at IB-UNICAMP with a scholarship from FAPESP.
“These are very expensive treatments, but we believe it could be a viable option because there aren’t as many critical patients as there were at the start of the pandemic, provided further research confirms that it’s safe for COVID-19 patients,” Moraes-Vieira said.
Murine coronavirus
The hypothesis tested in the study arose when Uruguayan researchers (co-authors of the article) observed that mice that did not express the protein TMEM176D responded more acutely to infection by MHV-A59. This protein regulates inflammasomes, protein complexes deployed by the innate immune system to trigger inflammation as a weapon against tumors, viruses and bacteria.
Inflammasome activation is more intense without TMEM176D. More inflammatory cytokines are released, including interleukin-1 beta (IL-1β), which is known to play a role in severe COVID-19 (more at: agencia.fapesp.br/34732/).
“Excessive release of IL-1β leads to T lymphocyte dysfunction, which we call T cell exhaustion,” Moraes-Vieira said. “These cells are so strongly activated that they can no longer respond adequately. This is common in chronic viral diseases like severe COVID-19, as we found in a study conducted early in the pandemic.”
An article on the study in question, published in 2020 in Cell Metabolism, is one of the most cited articles published in this journal in the last three years and motivated the Uruguayan team to propose a partnership (more at: agencia.fapesp.br/33296/).
In the trials involving mice, treatment with a PD-1 inhibitor restored T cell functionality. In addition, the researchers had access to blood from healthy donors and COVID-19 patients hospitalized at two institutions in Montevideo, Uruguay’s capital.
Experiments involving healthy cells infected with SARS-CoV-2 were conducted at UNICAMP's Laboratory of Emerging Virus Studies (LEVE) headed by Professor José Luiz Proença Módena, a co-author of the article, with support from FAPESP.
In trials involving human blood samples, only cells that came from patients in intensive care benefited from the administration of atezolizumab, a PD-1 inhibitor used in the study. This was because of inflammasome overactivation leading to exhaustion and dysfunction of adaptive immunity in these patients.
The findings should be considered with caution, the researchers warn. Studies involving cancer patients who were treated in this manner before contracting COVID-19 showed no benefits or pointed to negative results.
In one study, administration of the therapy before viral infection did not lead to an improvement in COVID-19. In another study, involving 423 patients, there were more cases of hospitalization and severe disease among those who had been given the inhibitor. On the other hand, a clinical trial of PD-1 inhibitors in sepsis patients showed the therapy to be safe. More research will therefore be needed to glean a deeper understanding of the effects of the treatment in the context of COVID-19.
The article “PD-1/PD-L1 blockade abrogates a dysfunctional innate-adaptive immune axis in critical β-coronavirus disease” is at: www.science.org/doi/10.1126/sciadv.abn6545.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





