
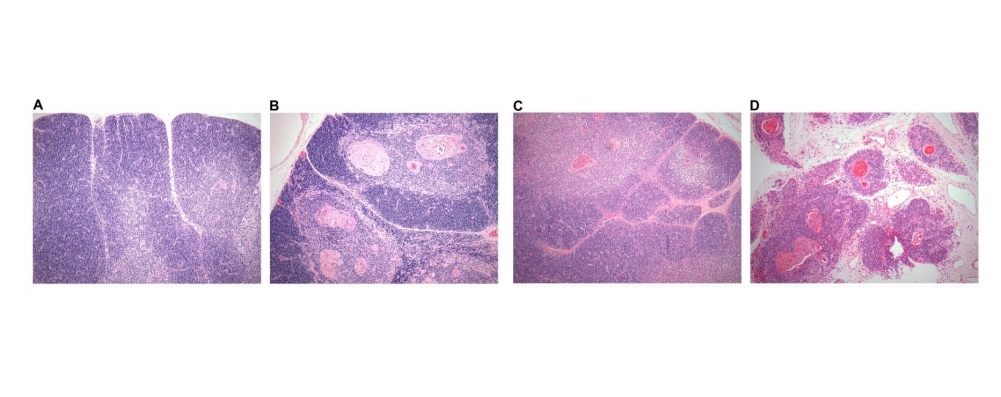
Analysis of whole thymic tissue samples showed that changes in gene co-expression networks cause primary immune deficiency in these patients (image: atrophied thymuses from infants with Down syndrome (B and D) compared with normal thymuses from children without Down syndrome (A and C); subjects were 9 months old in A and B, 18 months old in C and D)
Analysis of whole thymic tissue samples showed that changes in gene co-expression networks cause primary immune deficiency in these patients.
Analysis of whole thymic tissue samples showed that changes in gene co-expression networks cause primary immune deficiency in these patients.

Analysis of whole thymic tissue samples showed that changes in gene co-expression networks cause primary immune deficiency in these patients (image: atrophied thymuses from infants with Down syndrome (B and D) compared with normal thymuses from children without Down syndrome (A and C); subjects were 9 months old in A and B, 18 months old in C and D)
By Diego Freire | Agência FAPESP – Children with Down syndrome, a genetic disorder caused by abnormal cell division that results in an extra chromosome, suffer from more frequent infections and auto-immune diseases than children without the syndrome. The reason is primary immune deficiency, a congenital condition characterized by impaired functioning of the immune system. To achieve a deeper understanding of the processes underlying this condition, researchers at the University of São Paulo’s Medical School (FM-USP) in Brazil investigated the genomic dysregulation caused by the extra chromosome, which causes the immune cells of children with Down syndrome to attack the children’s own cells, making the defense cells less effective against invading microorganisms.
The researchers focused on the functioning of the thymus, an organ that science has explored relatively little, even though it plays an essential role in the immune system. Their work was part of the Thematic Project “Human thymus: development and diseases”, supported by FAPESP.
“A normal human cell has 46 chromosomes, of which 23 come from the father and 23 from the mother. Our chromosomes carry our DNA and all the information required by cells for development and reproduction,” said Carlos Alberto Moreira-Filho, a professor at FM-USP. “The cells of people with Down syndrome have 47, and the extra chromosome alters the functioning of various gene groups dispersed across the entire set of chromosomes. This causes dysregulation of the genome, which has to adapt to the presence of the extra chromosome in order to avert cell death. Functional rearrangement of the genome takes a different form in every tissue of a person with Down syndrome. It’s as if the organism had a Plan B that saves it from chaos but affects the development and functionality of several organs, including the thymus.”
The Greek word thymos refers to vital energy, and the thymus, located just behind the sternum, in the middle of the chest, indeed plays a vital role in the human immune system. Its primary function is the maturation of T lymphocytes (T cells), which both recognize and destroy cells that are damaged, cancerous or invaded by viruses or bacteria and which modulate the type and intensity of the organism’s immune response.
The researchers showed that the immunodeficiency of children with Down syndrome is closely related to genomic dysregulation of the thymus. In healthy individuals, when T cells leave the bone marrow, where they are produced, those lacking affinity for the individual's tissues as well as those capable of attacking these tissues are eliminated, leaving only T cells with the proper capacity to recognize the individual’s tissues and to identify alterations in this context. In individuals with Down syndrome, this mechanism malfunctions.
Moreira-Filho’s group has previously plotted the gene interaction networks associated with the alterations in functioning that occur in people with epilepsy. Now, they have used their expertise to understand what causes dysregulation of the thymus in Down syndrome, focusing on a comparison of thymic gene networks in individuals with and without the extra 21st chromosome.
“Among genes, as in networks of human relationships, there are those that perform very important functions, and others that play peripheral roles,” Moreira-Filho said. “In each type of human cell, about 20% of the genes have very important links to the rest. In other words, when they are altered in some way, the others ‘dance’ with them. Genes that lack this influence account for 80% [of all genes]. But the kinds of connections and which genes are responsible for this vary from one tissue to another. We produced a complete portrait of how the gene expression network rearranges itself in the thymus of a child with Down syndrome.”
The thymus of a baby born with Down syndrome shrinks drastically in the first two years of life, losing functional tissue and becoming infiltrated by fatty tissue. The thymus of an 18-month-old infant with Down syndrome is as atrophied as the thymus of an adult.
The researchers not only described how this process occurs at the cellular level but also defined how it relates to changes in gene networks. To do so, they first studied thymic expression of microRNAs, small non-coding RNA molecules that regulate gene expression.
In gene expression, the information contained in genes is used in the synthesis of a functional gene product, such as a protein. MicroRNAs control the robustness of gene expression and keep it at an appropriate level.
Having established the patterns of gene and microRNA expression, the researchers constructed gene co-expression networks and observed which molecules linked to which genes.
“We then found that most of the genes targeted by the microRNAs, especially those abundantly expressed, were different in the thymus of an individual with Down syndrome compared with a control, although in both cases, they were high-hierarchy genes, meaning they had many connections with other genes, “Moreira-Filho said. “The results pointed to the involvement of an epigenetic mechanism in the transition from the normal thymic gene interaction network to a ‘Plan B’ network. This epigenetic mechanism seems to contribute to the reorganization of gene functioning caused by the presence of an extra chromosome.”
Epigenetic processes are heritable changes in gene expression that arise not from intrinsic modifications of the underlying DNA sequence but from external or environmental factors.
e-Science
According to Moreira-Filho, the group’s studies of thymic gene co-expression in children with Down syndrome were possible only thanks to a collaboration with the Dante Pazzanese Institute of Cardiology in São Paulo.
“Very few research groups worldwide have access to thymus tissue from very young children with and without Down syndrome,” he said. “The thymus is located just above the pericardium, the tissue sac containing the heart, and has to be removed during heart surgery for blood vessel repair in infants, owing to its relatively large size in these patients. The institute’s donation of some of this precious material enabled us to assemble a large collection of thymic tissue samples from children with and without the extra chromosome.”
Another important collaborative contribution came from the University of São Paulo’s São Carlos Physics Institute (IFSC-USP), which developed the computational pipeline that enabled the researchers to identify gene communities in the cells of the samples they studied. This innovation was developed under the aegis of another Thematic Project supported by FAPESP: “Models and methods of e-Science for life and agricultural sciences”.
“Genes are like high-school classmates: they all belong to the same network of relationships, but some communicate more with each other than with the rest. In the case of genes, these communities reflect molecular and functional pathways,” Moreira-Filho explained. “The graphs generated by the mathematical and computational technique we developed show how these interactions work as well as their consequences, such as how community X interacts more with community Y or community F and which genes belong to each one.”
From the raw data on gene expression obtained in vitro, it was possible to “arrive at the sophistication of knowing not just who dances with whom but how everyone dances,” he added.
The conclusion was that the change to Plan B, adopted by genes in the presence of an extra chromosome, is largely controlled by microRNAs.
This is the first time that such an advanced level of complexity has been reached in the understanding of genomic dysregulation in the thymus, according to Moreira-Filho. The researchers also characterized gene co-expression in the normal thymus and are now analyzing it at different ages, with the aim of elucidating how it changes until the conclusion of age-related thymic involution. Their findings may help to clarify the molecular mechanisms of auto-immune and immunodeficiency diseases.
The article “Modular transcriptional repertoire and microRNA target analyses characterize genomic dysregulation in the thymus of Down syndrome infants,” by Carlos Alberto Moreira-Filho, Silvia Yumi Bando, Fernanda Bernardi Bertonha, Filipi Nascimento Silva, Luciano da Fontoura Costa, Leandro Rodrigues Ferreira, Glaucio Furlanetto, Paulo Chacur, Maria Claudia Nogueira Zerbini and Magda Carneiro-Sampaio, was published in Oncotarget and can be read at impactjournals.com/oncotarget.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.




