
Researchers synthesize tin oxide material with greater ability to detect nitrogen dioxide than existing chemical sensors used for this purpose
Researchers synthesize tin oxide material with greater ability to detect nitrogen dioxide than existing chemical sensors used for this purpose.
Researchers synthesize tin oxide material with greater ability to detect nitrogen dioxide than existing chemical sensors used for this purpose.

Researchers synthesize tin oxide material with greater ability to detect nitrogen dioxide than existing chemical sensors used for this purpose
By Elton Alisson
Agência FAPESP – Researchers from Universidade Estadual Paulista’s Chemistry Institute (IQ-Unesp Araraquara), in partnership with colleagues from the Department of Materials Science and Engineering at the Massachusetts Institute of Technology, have developed a material based on tin oxide (SnO) that has a higher sensitivity to nitrogen dioxide (NO2) than existing chemical sensors used to identify this highly toxic gas, formed by fuel reactions in the combustion engines of motor vehicles.
Developed through a FAPESP-funded project under the auspices of a partnership agreement with MIT, the material should result in a joint Unesp-MIT patent. The findings were described in the September edition of Sensors and Actuators B: Chemical.
“While the electrical resistance of pure materials used to detect nitrogen dioxide increased between 50 and 70 times in the presence of toxic gas, the sensor that we developed presents a 1,000-fold increase in resistance. This is the signal that we used to measure a sensor’s detection capacity,” said Marcelo Ornaghi Orlandi, faculty member at Unesp Ararquara’s Chemistry Institute and one of the authors of the study.
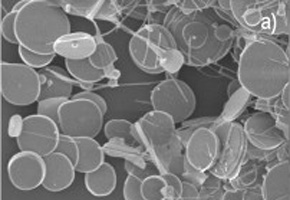
The material developed by the researchers consists of single crystalline tin oxide disks – similar to paper confetti – whose dimensions are on the micrometer scale.
The researchers used a process called carbothermal reduction to develop the material. The process was used to synthesize disks from tin oxide(II), instead of the traditional tin(IV) oxide (SnO2).
At the same time, they managed to establish the thermal and chemical stability and preserve the structure of the material and give it greater sensitivity to nitrogen dioxide than SnO2 – currently one of the most studied materials applied as a toxic gas sensor.
“Tin oxide is hard to synthesize because it is thermally unstable and tends to decompose at temperatures over 400 ºC,” explained Orlandi.
“Through rigorous control, we managed to synthesize [the material] for the first time and, at the same time, establish its thermal and chemical properties and increase its sensor response,” he affirmed.
Selectivity
Inside a chamber, researchers exposed the material to different types of toxic gases, including carbon gas (CO) and methane (CH4), in addition to nitrogen dioxide at temperatures between 100 ºC and 350 ºC.
Researchers observed that the material presents excellent selectivity and sensitivity toward nitrogen dioxide, mainly below 200 ºC – well below the material’s transition and decomposition temperature of 400 ºC.
At this temperature, the electrical resistance of the sensor increases 1,000-fold when the material is exposed to 100 parts per million of nitrogen oxide diluted in synthetic air, which simulates the Earth’s atmosphere.
Additionally, the tin oxide disk presented a selective capacity 100 times higher than that of other types of toxic gases, such as carbon gas and methane, said the researchers.
“We have not found another nitrogen dioxide sensor with such high selectivity and sensitivity in the scientific literature,” asserted Orlandi.
According to the researchers, one of the reasons the tin oxide disks present such high sensitivity and selectivity is that they are n-type semiconductors – materials such as zinc oxide (ZnO) and titanium oxide (TiO2) that present excess electrons in their structure and exhibit partial electric conductivity. Nitrogen dioxide has a predisposition to swap electrons with the material.
Thus, when nitrogen dioxide adheres to the surface of the tin oxide disks, the nitrogen dioxide molecules imprison free electrons and increase the electric resistance of the tin oxide disks.
“When the nitrogen dioxide adheres to the surface of tin oxide disks, it increases the material’s electrical resistance 1,000-fold, as we have measured and observed in the experiments,” said Orlandi.
By removing the nitrogen dioxide from the chamber where the tin oxide disks were placed and when the gas consequently left the surface of the material, the researchers found that the initial resistance of the chemical surface quickly returned to the same level observed prior to gas exposure.
“We found that the sensor is very specifically sensitive and selective to nitrogen oxide, very important characteristics for a sensor,” Orlandi affirmed. “If the sensor is selective and sensitive to all types of pollutant gases, it will not work for this purpose,” he added.
Virgin surface
Orlandi stressed that the 1,000-fold increase in the electrical resistance of the tin oxide disks, and thus the detection signal that they observed, was obtained without any alteration of the material’s surface – contrary to what must normally occur to increase the nitrogen oxide detection capacity of other types of existing chemical sensors based on tin(IV) dioxide.
Based on this finding, the researchers are making improvements to the surface of the disks to increase their electrical resistance when exposed to NO2.
“Instead of the 1,000-fold increase we obtained, we want to increase it to 2,000,” explained Orlandi.
“The signal of pure tin(IV) dioxide-based sensors is between 50 to [sic] 70, which is considered good,” he notes.
The researchers also developed tin oxide tape with dimensions on the nanometer scale, perceiving that the selectivity and sensitivity of the material toward nitrogen dioxide would be greater than that obtained on the micrometer scale.
By comparing the material on the two scales, however, the researchers found that the results presented by the micrometer-scale disks were better than those of the nanometer-scale tape.
According to Orlandi, this difference in the material’s performance arises because the disk has a more advantageous morphology compared to the nanotape. Because of their two large, parallel surfaces, the disks have a high concentration of unpaired electrons, which can be more easily transferred to nitrogen dioxide molecules.
“We believe that it is this surface material with a high concentration of unpaired electrons that leads to higher response in terms of selectivity and sensitivity to nitrogen dioxide on a micrometric scale than in the nanometric shape,” said Orlandi.
In the researcher’s estimation, the study corroborates the results of other international research published in the last few years, showing that many times, materials can exhibit better performance on the micrometer scale than on the nanometer scale.
Scientists still do not know, however, why the material is shaped like paper confetti. “The growth mechanism of this material is still unknown,” affirmed Orlandi.
MIT Partnership
The new sensor was developed in the laboratories of Unesp Ararquara’s Physical Chemistry Department.
Through the partnership established by Brazilian researchers with colleagues at MIT, Orlandi and student Anderson André Felix spent time at the U.S. university, where they conducted tests and evaluated the sensor properties of the new material. Felix is a FAPESP fellow currently pursuing a postdoctoral fellowship and is one of the authors of the study.
Through an analytical system available at MIT, in which eight material samples can be measured simultaneously at the same temperature and gas flow, the researchers found that the material’s capacity to detect nitrogen oxide was much greater than that of tin(IV) dioxide sensors.
Due to the exceptional performance exhibited by the new material, Professor Harry Tuller of MIT’s Materials Science and Engineering department concluded that the sensor should be patented and sent a request to MIT’s intellectual property office to begin the process.
“More than just an interesting scientific observation, it became clear for us that the material developed during this collaborative work could be a watershed in sensor technology,” said Tuller.
“At MIT, we are motivated to request patents on projects like these because this fulfills several objectives: it serves to raise awareness among scientists and engineers at the institution to think about how a development could impact society and to call industry’s attention to this new technological concept that could be unknown because they do not read or understand the scientific literature,” he explained.
The university filed a temporary patent at the United States Patent and Trademark Office.
In May of 2014, the request should become permanent in the United States, and afterward, the Unesp Innovation Agency (AUIN) should make a patent request at the National Institute of Intellectual Property in Brazil.
“In 2014, we should have patent requests for the material in the United States and Brazil,” said Fabíola Spiandorello, intellectual property manager at AUIN.
The article Giant Chemo-Resistance of SnO disk-like structures (doi: 10.1016/j.snb.2013.05.087) by Orlandi et al can be read in Sensors and Actuators B: Chemical at www.sciencedirect.com/science/article/pii/S0925400513006709.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.




