Discovery paves the way for the treatment of hereditary rickets
Study helps to uncover the mechanism that leads to X-linked hypophosphatemia, a genetic disease that affects bone mineralization and causes osteomalacia.
Discovery paves the way for the treatment of hereditary rickets
Study helps to uncover the mechanism that leads to X-linked hypophosphatemia, a genetic disease that affects bone mineralization and causes osteomalacia.
Study helps to uncover the mechanism that leads to X-linked hypophosphatemia, a genetic disease that affects bone mineralization and causes osteomalacia
By Karina Toledo
Agência FAPESP – A study conducted at Universidade Federal de São Paulo (Unifesp) in collaboration with McGill University in Canada should pave the way for the treatment of the most common form of hereditary rickets: X-linked hypophosphatemia (XLH).
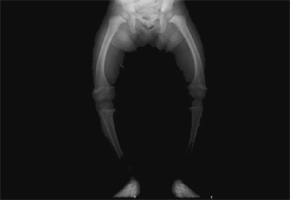
The genetic disease causes an uncontrolled loss of phosphate through urine, which leads to problems in bone mineralization, osteomalacia (bone softening), and deformation of the skeleton and could result in reduced height, spontaneous dental abscesses, weakness and muscular pain. The problem affects one in 20,000 people and is normally diagnosed in childhood.
“In 1995, scientists discovered that XLH was the result of a mutation in a gene called PHEX, which encodes an enzyme of the same name. But until now, no one knew the role of this enzyme or how its function could set the disease in motion,” explained Nilana Barros, Unifesp professor and coordinator of the
FAPESP-funded study.
In experiments conducted with mice that carry the same genetic mutation as found in patients with XLH (Hyp mice), the group showed for the first time that the PHEX enzyme is responsible for degrading a protein called osteopontin.
“Osteopontin has a potent inhibitory action on mineralization in our bones. It must be present in regulated quantities because tissue should not constantly mineralize . But in the bones of Hyp mice, we observed intense accumulation of osteopontin and specific fragments of this protein . This means that mineralization is constantly being inhibited, preventing the ‘hardening’ of bones,” affirmed Barros.
In in vitro tests, the researchers showed that the PHEX enzyme was capable of completely degrading osteopontin to the point of deactivation. “Based on this discovery, it is possible to develop an enzymatic replacement therapy to treat carriers of XLH in a more efficient manner,” commented Barros.
Currently, according to Barros, the standard treatment consists of administering high doses of phosphate and calcitriol (a form of vitamin D), but the results are not satisfactory. “Several studies have shown that growth in children is corrected in many cases, but orthopedic procedures are necessary to resolve leg deformities. Furthermore, therapy is associated with kidney complications. For this reason, adult patients often receive treatment only when they are affected by pseudofractures or constant bone pain,” she explained.
Opening doors.
During her post-doctoral studies, which were conducted under the supervision of Professor Adriana Karaoglanovic Carmona of Unifesp and through a
FAPESP fellowship, Barros began to suspect that osteopontin was the missing piece of the XLH puzzle.
“The scientific literature pointed to other molecules as candidates for the PHEX substrate, but the more I studied, the more I believed that biochemically, there was an affinity between this enzyme and osteopontin. It was a combination of science, dedication, persistence and luck,” said Barros.
It took four years of intense work in collaboration with Carmon and Professor Marc Mckee, a specialist in bone disease at McGill University and coordinator of research in Canada, for her suspicions to be confirmed. The results will be published in March in the Journal of Bone and Mineral Research, the main scientific journal in the area.
In the early experiments, the group investigated the interaction between PHEX and osteopontin only in bone, the main tissues affected in XLH carriers. But the experiments have continued, and now, the group is studying the systemic impact of osteopontin accumulation in the organism and its influence on the regulation of phosphate.
“It is a multifunctional molecule. It has some variations and could have different functions in each of the tissues in which it is found,” said Barros. For example, osteopontin also exists in above-normal quantities in people with cancer, even those without the genetic mutation that causes XLH.
“The more aggressive the tumor, the greater the quantity of osteopontin. Our group also discovered that the PHEX enzyme is found in tumors, and we are now attempting to discover why it does not regulate osteopontin in this case,” she said.
According to Barros, the scientific literature indicated that PHEX was found in bones and teeth. “But we are seeing that this enzyme is also crucial for modulating osteopontin in other tissues,” she said.







