
The safety of cellular therapy for age-related macular degeneration will be evaluated in 2013
The safety of cellular therapy for age-related macular degeneration will be evaluated in 2013.
The safety of cellular therapy for age-related macular degeneration will be evaluated in 2013.

The safety of cellular therapy for age-related macular degeneration will be evaluated in 2013
By Karina Toledo
Agência FAPESP – Scientists will begin to test a stem cell-based therapy for age-related macular degeneration (AMD) – the main cause of blindness in the elderly – in humans beginning in early 2013.
Pete Coffey, a British scientist at University College London, made the announcement during the 7th Brazilian Congress on Stem Cell and Cellular Therapy, which was held in São Paulo and sponsored by FAPESP.
The British study is being conducted under the auspices of the London Project to Cure Blindness, a partnership between Coffey and surgeon Lyndon da Cruz of the Moorfields Eye Hospital in London.
The technique, which consists of applying a type of Band-Aid containing embryonic stem cells to the affected area of the retina, has been tested successfully in rats, mice and pigs.
“Now we will conduct tests with 10 patients, a small and very specific group initially, because the objective is to evaluate the safety of the treatment,” Coffey explained in an interview with Agência FAPESP.
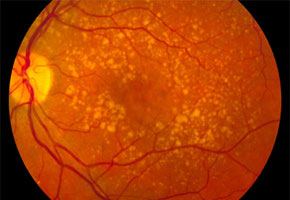
According to Coffey, AMD attacks the central region of the retina, known as the macula, where there is a large concentration of photoreceptors, which are responsible for the perception of color and details. Below this layer of photoreceptors is the retinal pigment epithelium and, farther below, the Bruch membrane.
In predisposed individuals, cellular debris begins to form crystals, known as drusen, in the back of the eyes with increasing age, and these drusen destroy photoreceptors and provoke the proliferation of abnormal blood vessels below the retina. This blood vessel proliferation affects the integrity of the macula and compromises the individual’s central vision and ability to distinguish colors.
This disease is common in patients over age 55 and affects more than 25% of people over 75. Roughly 90% of cases are the dry form of AMD, which evolves slowly and has no treatment.
The remainder of cases are the wet form of the disease, which is much more aggressive and is characterized by hemorrhages that compromise the retinal tissues. Treatment consists of the use of lasers or the injection of drugs that inhibit the formation of new blood vessels in this region.
“In the most severe cases, the intermediary layer of the retina (pigment epithelium) breaks, resulting in a loss of vision at this point. These are the cases we intend to treat,” explained Coffey.
Initially, the British team developed a surgical technique that involves removing healthy cells from the retinal pigment epithelium of the patient and transplanting them into the affected region. “We had good results, but the surgery is lengthy, lasting up to three hours, which increases many risks,” explained Coffey.
To reduce the duration and level of risk of the surgery – and to benefit patients with less advanced stages of the disease – the scientists decided to apply a pre-prepared membrane containing pigment epithelial cells obtained from embryonic stem cells to the intermediary layer of the retina.
“In tests with animals, we did not register any tumor formation because the process of cellular differentiation also occurs in labs. If the therapy proves to be safe in humans and we manage to maintain good vision in three or four of the 10 patients, the clinical trials will be considered very successful,” commented Coffey.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.




