

In a preclinical trial involving mice, antibodies recognized all three variants of the target protein, completely preventing infection in some cases (photo: CT-Vacinas/UFMG)
USP is developing the vaccine in partnership with the Vaccine Technology Center at the Federal University of Minas Gerais (UFMG). Permission to conduct a clinical trial is due before the end of this month.
USP is developing the vaccine in partnership with the Vaccine Technology Center at the Federal University of Minas Gerais (UFMG). Permission to conduct a clinical trial is due before the end of this month.
In a preclinical trial involving mice, antibodies recognized all three variants of the target protein, completely preventing infection in some cases (photo: CT-Vacinas/UFMG)
By Luciana Constantino | Agência FAPESP – Brazilian scientists have filed for a patent on a vaccine against the most common type of malaria in the Americas, and plan to apply in January 2025 for permission to conduct a Phase 3 clinical trial. The Plasmodium vivax malaria vaccine candidate has already passed preclinical trials to assess quality, efficacy and safety with promising results.
Malaria is caused by five species of the parasitic protozoan Plasmodium, three of which are found in Brazil (P. vivax, P. falciparum and P. malariae). The parasites are transmitted to humans by bites of infected female Anopheles mosquitoes. The only vaccine in use is against P. falciparum. Administration of this vaccine to children in some sub-Saharan countries has been recommended by the World Health Organization (WHO) since 2021.
“Our product is unique in the world and entirely made in Brazil. My aim since the start of our research over ten years ago has been to produce a vaccine. We’re now in the final stage for authorization of clinical trials,” Irene Soares, co-principal investigator for the research project and full professor at the University of São Paulo’s School of Pharmaceutical Sciences (FCF-USP), told Agência FAPESP. The other PI is Ricardo Gazzinelli, a professor at the Federal University of Minas Gerais (UFMG), director of UFMG’s Vaccine Technology Center (CT-Vacinas), and head of the National Science and Technology Institute for Vaccines (INCT-Vacinas).
Soares receives support from FAPESP via a Thematic Project. The group received federal funding from the National Council for Scientific and Technological Development (CNPq, INCT-Vacinas 465293/2014-0) for initial proof-of-concept studies, and from the Brazilian Innovation Agency (FINEP, agreement 01.22.0046.00) for the Phase 1 clinical trial.
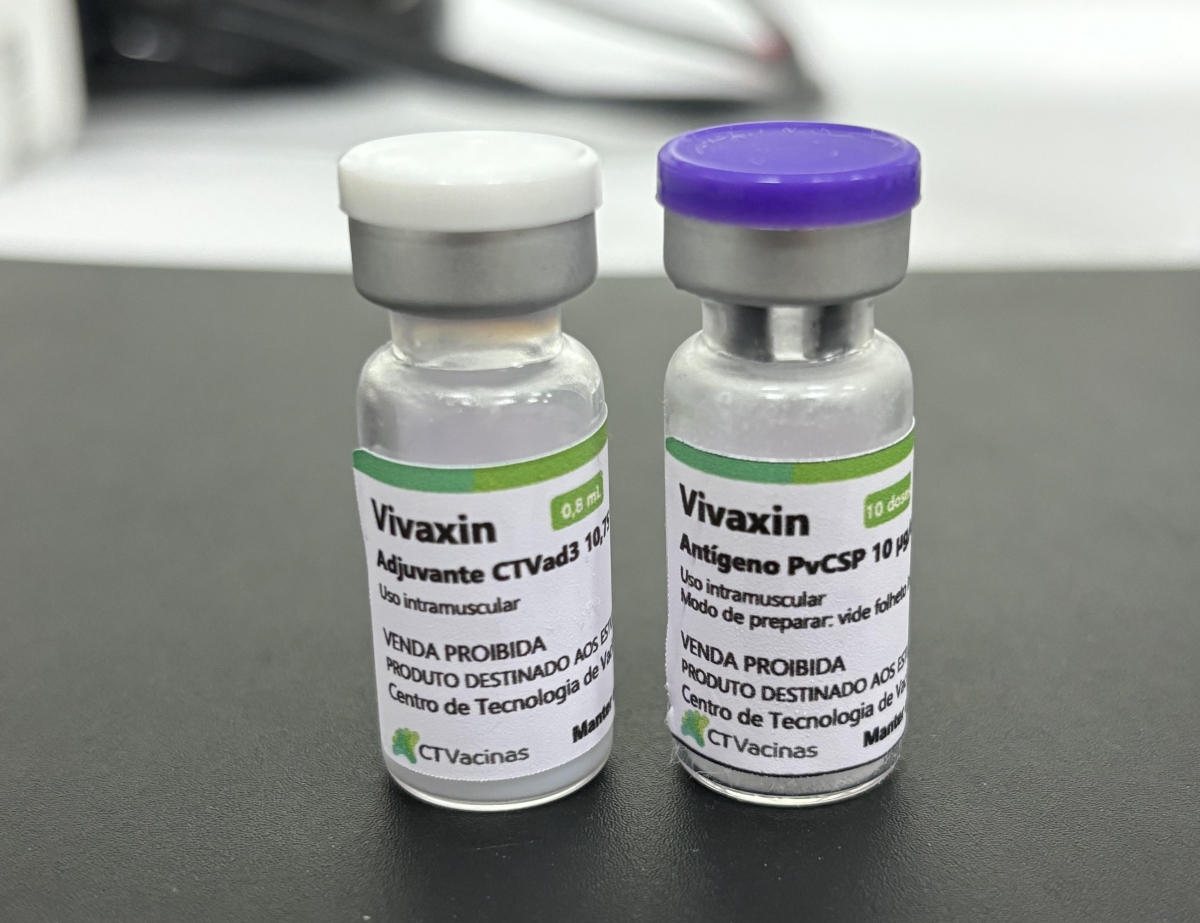
The vaccine is called Vivaxin, and has passed tests to certify good laboratory practices (GLP) and good manufacturing practices (GMP). It was presented in September by UFMG’s CT-Vacinas, which is partnering with FCF-USP to develop the product, during the Second Innovation and Sustainability Congress hosted by the Belo Horizonte Technology Park (BH-TEC).
“There’s a yawning gap in vaccine research lines in Brazil, as was evident during the COVID-19 pandemic. Basic research, typically done at universities, involves selecting antigen and adjuvant, and conducting a proof-of-concept trial. This results in publication of articles, but the research stops there. It doesn’t get as far as production of a vaccine. The aim of our partnership is to overcome this ‘valley of death’ and get to a final product, with production and testing in humans all done in this country, a rarity in Brazilian vaccine science,” Soares said.
The patent application was filed in late October by the University of São Paulo’s Innovation Agency and UFMG’s Center for Technology Transfer and Innovation, protecting the production process and the final formulation with the adjuvant developed by the team at CT-Vacinas. The results of the latest tests will be published shortly in a scientific journal.
According to an article by the research group published in the journal Vaccine in April 2024, the vaccine induced high levels of antibodies in mice and rabbits, proving safe and well tolerated in preclinical trials. The formulation combines three alleles (alternative genetic forms) of P. vivax circumsporozoite protein, or PvCSP, in a single molecule with the aim of enhancing the vaccine’s efficacy against all allelic variants.
Unlike P. falciparum (most prevalent in Africa), P. vivax has a circumsporozoite protein with three alleles: VK210, VK247, and P. vivax-like. It was chosen as a target because it is the most abundant surface protein in Plasmodium sporozoites, the motile form of the parasite present in the mosquito’s saliva gland. The sporozoites actively migrate in the host’s dermis and enter blood vessels to infect the liver by binding to hepatocyte receptors.
In the study with the novel formulation, antibodies produced by the vaccinated mice recognized all three variants. In some cases, infection was completely prevented (sterile protection), while in others the appearance of parasites in the bloodstream was delayed.
In recent years, the scientists have tested several adjuvants (components that enhance the vaccine’s immunogenicity when administered in conjunction with an antigen). One of these tests resulted in another article, published in Frontiers in Immunology, also in April 2024, with FAPESP’s support (projects 14/18102-7, 18/14933-2 and 17/11931-6).
Malaria in Brazil and the world
Considered endemic in the Amazon region and a global public health problem, malaria causes high fever, shaking chills, sweating, and headache. In severe cases, it can cause seizures, bleeding, and impaired consciousness. Outpatient treatment is the norm, and the SUS (Sistema Único de Saúde, Brazil’s public health service) supplies free pills.
In 2024, 117,946 cases of malaria were reported between January and October, and 80% (95,113) were caused by P. vivax, according to the Ministry of Health.
The disease is spreading at an alarming rate among Indigenous communities, with 45,100 cases being reported in the first ten months of the year, or 12% more than in the corresponding period of 2023.
On World Malaria Day (April 25), the Pan American Health Organization (PAHO) called on governments to step up efforts to tackle the disease, which disproportionately impacts Indigenous communities, migrants, and other vulnerable groups.
Some 480,000 cases were reported in the Americas in 2023. While the number of reported cases has declined since 2017, when the total reached a peak of 934,000, some countries are still far from achieving the target of a 75% reduction by 2025, set by the World Health Organization (WHO).
The article “Non-clinical toxicity and immunogenicity evaluation of a Plasmodium vivax malaria vaccine using Poly-ICLC (Hiltonol®) as adjuvant” is at: www.sciencedirect.com/science/article/abs/pii/S0264410X24002299?via%3Dihub.
The article "Poly I:C elicits broader and stronger humoral and cellular responses to a Plasmodium vivax circumsporozoite protein malaria vaccine than Alhydrogel in mice” is at: www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1331474/full.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





