

Experiments were conducted on mice by a group at the University of São Paulo. The results, reported in the journal Endocrinology, could have future implications for therapies to control body weight (image: researchers’ archive)
Experiments were conducted on mice by a group at the University of São Paulo. The results, reported in the journal Endocrinology, could have future implications for therapies to control body weight.
Experiments were conducted on mice by a group at the University of São Paulo. The results, reported in the journal Endocrinology, could have future implications for therapies to control body weight.
Experiments were conducted on mice by a group at the University of São Paulo. The results, reported in the journal Endocrinology, could have future implications for therapies to control body weight (image: researchers’ archive)
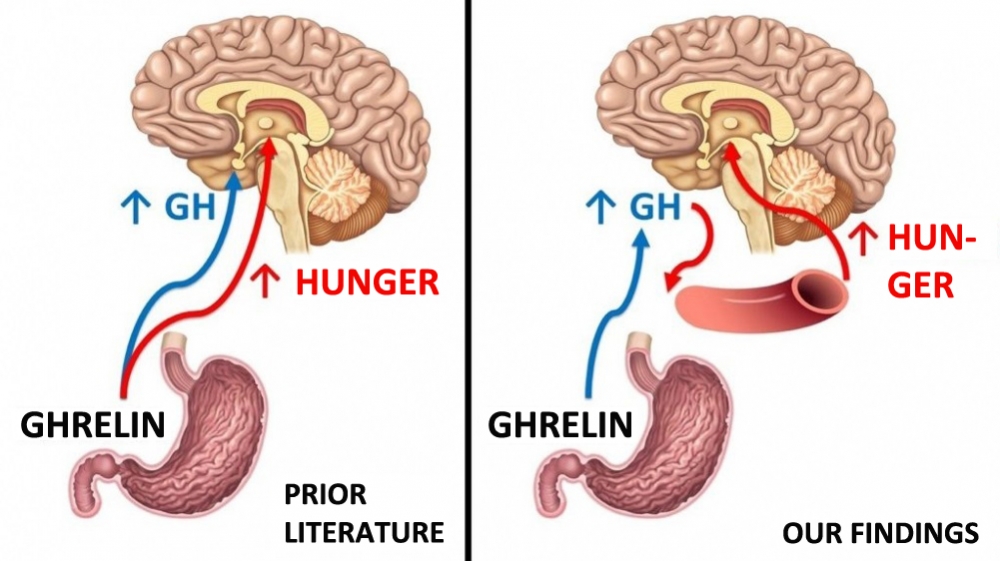
By Luciana Constantino | Agência FAPESP – A study involving mice has shown how growth hormone (GH) acts on the brain and plays a key role in stimulating appetite, in addition to the functions scientists had previously discovered. GH regulates the capacity of ghrelin to promote an increase in food intake. Ghrelin is often referred to as the “hunger hormone”.
The study was conducted at the University of São Paulo’s Biomedical Sciences Institute (ICB-USP) in Brazil. According to its findings, ghrelin acts on the pituitary gland to trigger the release of GH. Both hormones have receptors in the hypothalamus, a region at the base of the brain with several functions including appetite regulation.
“The hunger stimulator label should at least be shared between ghrelin and GH, because without the effect of stimulating GH secretion, ghrelin also loses the capacity to stimulate the appetite,” said José Donato Junior, a researcher at ICB-USP and last author of an article on the study published in the journal Endocrinology.
FAPESP supported the study via two postdoctoral scholarships (16/20897-3 and 17/25281-3), and a Thematic Project (20/01318-8).
“Our discoveries can have future implications for therapies to control body weight and regulate food intake,” Donato told Agência FAPESP.
He recalled that in 2019 another research group showed that the higher the level of GH, the more the brain produces AgRP, a peptide that is a powerful appetite stimulator. The study involved patients with acromegaly, a chronic disorder due to a malfunctioning pituitary, which produces too much GH and causes enlargement of the hands, feet and face. It was based in part on the findings of another study conducted at ICB-USP showing how GH acts directly on the brain to conserve energy when body weight falls, in addition to its role in linear bone growth and skeletal maturation (more at: agencia.fapesp.br/30192).
Mechanism
In the study reported in Endocrinology, the researchers at ICB-USP investigated the response to ghrelin in mice genetically modified so as not to have the GH receptor specifically in neurons. Their GH rose normally after ghrelin was injected, but they did not display the expected response to appetite stimulation. They were also found to have reduced hypothalamic levels of hunger-stimulating neuropeptides, such as NPY. The conclusion was that the action of GH in the murine brain was necessary for ghrelin to stimulate food intake.
Ghrelin is the only hormone linked to appetite. It is produced in the stomach. Other hormones produced in the gut or adipose tissue typically cause a feeling of satiety. Discovered in 1999, ghrelin is also associated with stress, which explains why hunger can increase in tense or challenging situations.
GH was discovered in the 1950s and is still seen as the most important factor in body growth. A deficiency of GH causes dwarfism, a condition in which the person’s stature is abnormally short for their age and sex (typically around 1.40 m). Excessive GH leads in childhood to gigantism (now less common thanks to the availability of treatment in infancy) and in adulthood to acromegaly.
Next steps
According to Donato, the research group’s next steps will focus on understanding better how GH acts in the brain. “This takes us toward therapeutic proposals,” he said. “We’re testing a drug used in cases of acromegaly to block the action of ghrelin. The problem is the possible side-effects, so we must understand the cellular mechanisms used by GH to affect neurons.”
The ICB-USP group has also published a review of the literature in Frontiers in Neuroendocrinology, with information on how the hypothalamus (the brain region that controls food intake and metabolism) regulates or is regulated by physical exercise. This study also showed that a combination of physiological and molecular approaches can explain the physiology of exercise in terms of weight loss, adherence to training and performance.
The article “Ghrelin-induced food intake, but not GH secretion, requires the expression of the GH receptor in the brain of male mice” is at: academic.oup.com/endo/article-abstract/162/7/bqab097/6273366.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





