

Immunofluorescence images of head and neck tumor cells. The left-hand column shows drug-sensitive and resistant cells treated with cetuximab. The images in the middle column show the location of the receptor blocked by the drug in sensitive and resistant cells. The merged images in the right-hand column show cetuximab (left) and the location of the receptor (center) in the sensitive and resistant cells (image: Izabela Faria Gomes et al/Cells)
Cetuximab is one of the few drugs approved for this type of cancer, but it is expensive and effective only for 40% of patients. An article in the journal Cells describes molecular alterations observed in tumor cells that indicate resistance to the drug, paving the way to the development of a predictive test.
Cetuximab is one of the few drugs approved for this type of cancer, but it is expensive and effective only for 40% of patients. An article in the journal Cells describes molecular alterations observed in tumor cells that indicate resistance to the drug, paving the way to the development of a predictive test.
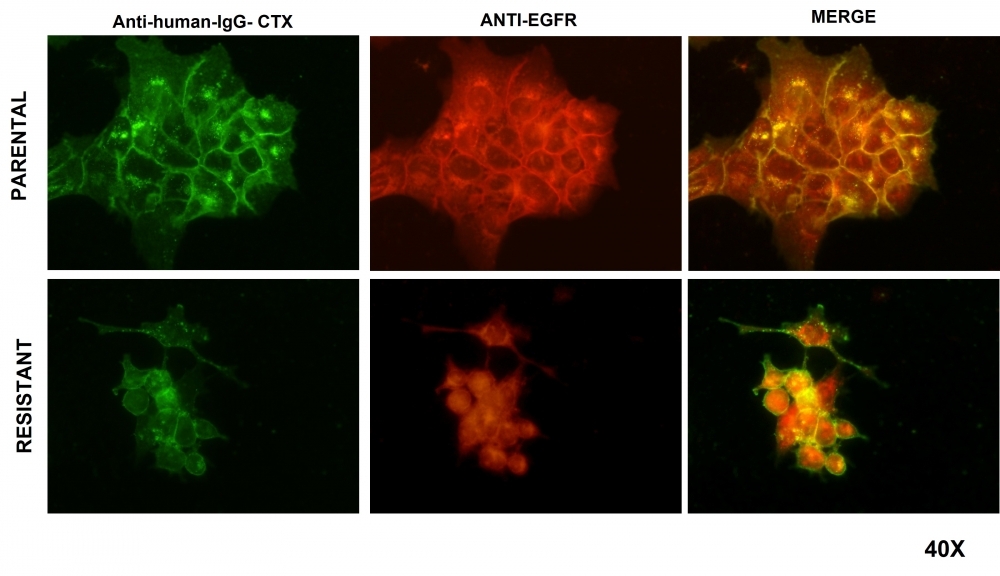
Immunofluorescence images of head and neck tumor cells. The left-hand column shows drug-sensitive and resistant cells treated with cetuximab. The images in the middle column show the location of the receptor blocked by the drug in sensitive and resistant cells. The merged images in the right-hand column show cetuximab (left) and the location of the receptor (center) in the sensitive and resistant cells (image: Izabela Faria Gomes et al/Cells)
By André Julião | Agência FAPESP – A group of researchers affiliated with institutions in Brazil and Portugal has discovered a number of molecular alterations that serve as biomarkers of resistance to cetuximab, one of the few medications approved for use in treating head and neck cancer. Some 60% of patients do not respond well to the drug.
An article on the study is published in the journal Cells. The findings are expected to help physicians predict whether patients will respond to treatment with cetuximab, which is expensive.
The authors recommend clinical trials to confirm their conclusion that combining cetuximab with other medications is a successful strategy to overcome resistance to the drug.
“The treatment of head and neck tumors has evolved relatively little. It still basically comprises surgery, radiation therapy and chemotherapy. Cetuximab is revolutionary because it specifically targets a cellular receptor known by the acronym EGFR that is significantly altered by these tumors,” said Rui Manuel Reis, last author of the article. Reis is a researcher at Hospital de Amor (formerly Barretos Cancer Hospital) in Barretos, São Paulo state, Brazil, and at the University of Minho in Portugal.
The cause of cetuximab resistance in some patients with head and neck cancer was unknown until now. This is a decisive factor in deciding how to treat the disease. Because of the uncertainty about whether this expensive drug would work, it was not included in the list of cancer drugs covered by the SUS (Sistema Único de Saúde, Brazil’s national health system).
Cetuximab is used in personalized medicine to treat metastatic colorectal cancer. Given the existence of genetic tests for mutations in the genes KRAS, NRAS and BRAF, which predict resistance to the drug, it is administered only to patients with the potential to respond positively. The study on head and neck cancer paves the way for the development of a similar approach to personalized treatment for this kind of tumor.
“We analyzed resistant cell lines at the level of DNA, RNA and proteins, and found some biomarkers that were overexpressed, such as the protein mTOR. Our proposal is to reverse this resistance phenotype with a combination of cetuximab and drugs that inhibit this and other overexpressed proteins,” said Izabela Faria Gomes, first author of the article. The study was part of her PhD research at Hospital de Amor with a scholarship from FAPESP.
Specific inhibitors of the mTOR protein and pathway are currently available on the market, so that expression of the protein by resistant cells can be inhibited when they are combined with drugs that act on other proteins in the same cellular signaling pathway.
Resistant cell lines
To discover the mechanisms behind responses to the drug, the Barretos research group developed an in vitro model that mimicked the resistance that occurs in head and neck tumors. They cultured a tumor cell line initially sensitive to cetuximab and “bombarded” it with the drug for a year. They then analyzed the cells that survived and therefore proved resistant to the drug, using an array of molecular tools to understand what made them different.
“We brought selective pressure to bear on this tumor cell line. The longer it was exposed to the drug, the more resistant it became and the more visible the molecular alterations,” said Renato da Silva Oliveira, second author of the article. Oliveira developed the method during his PhD research at Hospital de Amor’s Center for Molecular Oncology under Reis’s supervision.
The resistance model created by the researchers is being refined in partnership with the Nuclear and Energy Research Institute (IPEN) in Brazil and the University of Alabama in the United States.
In future, it could serve as a basis for a specific test to detect head and neck tumor resistance to the drug. If it progresses thus far, the study may be conducted in partnership with A.C. Camargo Cancer Center in São Paulo, where some patients are treated with cetuximab.
The next step is validation of the findings in animal models and in patients who prove resistant to treatment with cetuximab. In addition, future studies could test combinations of cetuximab with other therapeutic agents.
The article “Comprehensive molecular landscape of cetuximab resistance in head and neck cancer cell lines” is at: www.mdpi.com/2073-4409/11/1/154/htm.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





