
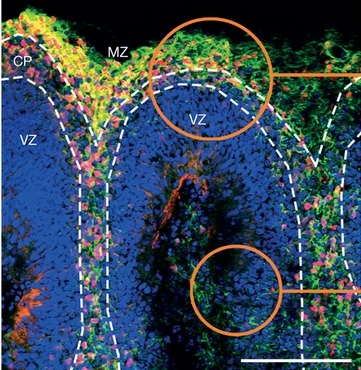
Experiments with mice described in Nature confirm that Zika virus crosses the placental barrier to infect and kill fetal brain cells. In vitro trials show the Brazilian strain is more aggressive than the original African strain (image of human cerebral organoid, showing the marginal zone (MZ), cortical plate (CP) and ventricular zone (VZ))
Experiments with mice described in Nature confirm that Zika virus crosses the placental barrier to infect and kill brain cells.
Experiments with mice described in Nature confirm that Zika virus crosses the placental barrier to infect and kill brain cells.

Experiments with mice described in Nature confirm that Zika virus crosses the placental barrier to infect and kill fetal brain cells. In vitro trials show the Brazilian strain is more aggressive than the original African strain (image of human cerebral organoid, showing the marginal zone (MZ), cortical plate (CP) and ventricular zone (VZ))
By Karina Toledo | Agência FAPESP – A study conducted by scientists belonging to the Zika Network, which FAPESP supports, published on May 11, 2016 by the journal Nature presents definitive evidence that infection by Zika virus during pregnancy can cause congenital brain malformation, confirming a link with the 2015 outbreak of microcephaly in Brazil.
The lead authors of the study are researchers at the University of São Paulo (USP) in Brazil. They demonstrated in experiments with mice that Zika virus crossed the placental membrane to infect and kill future brain cells of the murine fetuses in gestation.
In vitro trials also suggest that the Brazilian strain of Zika virus is more aggressive than the African strain that originally infected monkeys. This corroborates the theory that recent mutations have made the virus more effective at infecting humans (read more at http://agencia.fapesp.br/22466).
“There’s no longer any doubt that Zika virus is neurotoxic and can cause microcephaly,” said Jean Pierre Peron, a professor at USP’s Biomedical Science Institute (ICB) and one of the authors of the paper. “The birth defect we found in the brains of these mouse pups, characterized mainly by a reduction in the thickness of the cortex, is very characteristic and also found in human newborns. In addition, we found that in the brains of these mouse pups, the virus replicated in far greater quantities than in other organs.”
Some of the findings are based on experiments with SJL mice, which Peron said were considered a suitable model for research on Zika. Females were infected between the tenth and twelfth day of pregnancy with a viral strain isolated from a baby born with microcephaly in Paraiba State in the Northeast of Brazil in 2015.
Whole-body growth delay or intra-uterine growth restriction was observed in the pups exposed to Zika virus immediately after birth. Pups born to control females (not infected) weighed 3.4 g at birth on average, compared with 1.4 g for infected pups. The skull length and height were at least one third smaller in the infected group.
Microscopic analysis of the brain tissue showed reduced cortical layer thickness and alterations in the number and morphology of cortical cells, according to Patricia Beltrão-Braga, a researcher at USP’s School of Veterinary Medicine & Animal Science (FMVZ).
“Histopathological analysis revealed abnormal cell morphology, mainly in the cortex but also in the hypothalamus and thalamus,” she said. “The cells displayed what we call intranuclear vacuoles, meaning that the chromatin was clumped together in one small portion of the nucleus, which at first sight appeared to be empty.”
This type of morphological abnormality is frequently seen in processes that lead to cell death, according to the researchers. Indeed, gene expression analysis performed at a later stage of the study showed that genes associated with apoptosis (programmed cell death) and autophagy (intracellular degradation and recycling of dysfunctional components) were overexpressed in pups exposed to Zika virus compared with those in the control group.
To supplement the in vivo experiments, the researchers subjected brain, kidney, liver and spleen tissue from the pups to real-time polymerase chain reaction (PCR) tests designed to detect viral RNA during the acute phase of infection.
“We found that the virus replicated in the spleen, liver and kidney, but far less so than in the brain,” Person said.
“Taken in aggregate, the results show that the virus has an enormous preference for the cells of the nervous system,” Beltrão-Braga said. “We detected not just a larger amount of viral RNA but also the main effects of infection in the brain.”
Curiously, the same results were not observed in the first tests performed at USP with C57BL/6 mice, which are more often used in laboratories.
“This strain of laboratory mouse is known to display a more robust immune response, producing more alpha and beta interferon cytokines,” Peron explained. “We believe that this may have enabled the animals to eliminate the virus more effectively and prevent it from crossing the placental barrier. Our tests used different doses of the virus on different infection dates, and in no case were the murine pups born with any type of malformation.”
For Peron, this finding evidences the influence of the mother’s genome on the extent of the damage done to the fetus by Zika virus. “As in mice, there must certainly be human mothers who are more susceptible to the virus and others who are more resistant,” he said. “Now we need to do more research on the mechanisms involved.”
Mini-brains
The lab tests involved three different models of human cells: two-dimensional cultures (on glass plates) of neurons and neural progenitor cells (a type of stem cell capable of differentiating into neurons and glial cells); three-dimensional cultures of neural progenitors (cultured in suspension to form neurospheres); and cerebral organoids or mini-brains (millimetric three-dimensional structures created in the lab from human induced pluripotent stem cells, capable of mimicking first-trimester fetal brains).
All three models were infected with the Brazilian and African strains of Zika virus as well as yellow fever virus.
“We decided to use yellow fever virus, which is a member of Flaviviridae, the same family as Zika and dengue, as a kind of negative control to be sure that the effects observed were not due to any viral infection but were specific to Zika,” Beltrão-Braga explained.
In the experiment with 2D cultures, all three viruses were capable of infecting both neurons and neural progenitor cells, and only the culture exposed to yellow fever virus displayed no cell death. A comparison of the two cultures infected with Zika showed many more deaths of neural progenitor cells than neurons.
According to Peron, one of the possible explanations for the relative susceptibility of neural progenitors is that they express larger amounts of TYRO3, AXL and MERTK (TAM) receptors, an important family of membrane receptors. Previous research showed that TAM receptors facilitate human cell invasion by dengue and Zika.
In the assays performed on neurospheres, which consisted solely of neural progenitors, microscopic analysis clearly showed the damage caused by Zika, especially the Brazilian strain.
The uninfected neurospheres used as controls doubled in size over a four-day period, while the neurospheres infected with the African strain grew very little, pointing to cell death, and those infected with the Brazilian strain shrank to less than a third of their initial size, displaying a disorganized structure that is also considered evidence of cell death.
The greater the quantity of viruses in the medium, the more cell destruction was observed. For Beltrão-Braga, this may explain the occurrence of malformations with differing degrees of severity among babies born with microcephaly in Brazil.
Finally, cell death in the mini-brains infected with the Brazilian strain was found to be concentrated in areas where neurogenesis (the production of new neurons) was greatest, and some cellular subtypes were practically exterminated after four days.
The organoids infected with the African strain did not display significant differences compared with the mini-brains exposed to yellow fever virus. In already differentiated areas, such as the area from which the cortex would grow, neurons and neural progenitor cells died from infection by both the Brazilian and African strains.
Completing the in vitro analysis, the researchers compared the effects of infection by the Brazilian strain on human mini-brains and organoids grown from chimpanzee pluripotent cells.
“The results show that the Brazilian strain is so different from the African original that it’s no longer able to infect chimp organoids, although the similarity between the human genome and the chimp genome is 99%,” Beltrão-Braga said. “Zika virus appears to have undergone mutations in recent years that have made it more ‘humanized’ and capable of causing the malformations we’re seeing in Brazilian newborns.”
Next steps
According to Peron, by showing that SJL mice are a good model in experiments involving infection by Zika virus, the study has paved the way for research designed to develop vaccines and methods for protecting the babies of infected mothers.
“My lab’s main interest at the moment is to understand the biology behind the virus’s interaction with TAM receptors and why it causes massive neural progenitor cell death. We want to find out which pathways are activated by the virus’s interaction with these receptors,” Peron said.
In addition to investigating why neurons are less affected than their progenitors, Beltrão-Braga is also interested in discovering ways to avoid cell death induced by the virus. “We need to know what type of immune response is triggered in the brain and whether it favors or minimizes cell death,” he said.
The co-authors of the paper published by Nature included Paolo Zanotto (ICB-USP), coordinator of the Zika Network, and Alysson Muotri, a researcher at the University of California San Diego in the United States. The analysis was mostly performed by Fernanda Cugola, Isabella Fernandes and Fabiele Russo, graduate students at FMVZ-USP.
“The Brazilian Zika virus strain causes birth defects in experimental models” (doi:10.1038/nature18296) by Fernanda R. Cugola et al. can be read at www.nature.com/nature/journal/vnfv/ncurrent/full/nature18296.html.
Videos of USP’s researchers who participated in the study can be watched (in Portuguese, with English subtitles available) at: www.youtube.com/playlist?list=PLwA0zWYFcS_g1FxdtBz5OyaiIqdaLpbQv.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





