

Brazilian researchers use an editing tool to investigate a gene that plays a key role in eliminating autoaggressive cells and controlling the development of diseases such as type 1 diabetes (image: immunofluorescence image of mTECs, with cell nucleus highlighted in blue and Aire genes shown as red dots / Karina F. Bombonato-Prado, FORP-USP)
Brazilian researchers use an editing tool to investigate a gene that plays a key role in eliminating autoaggressive cells and controlling the development of diseases such as type 1 diabetes.
Brazilian researchers use an editing tool to investigate a gene that plays a key role in eliminating autoaggressive cells and controlling the development of diseases such as type 1 diabetes.
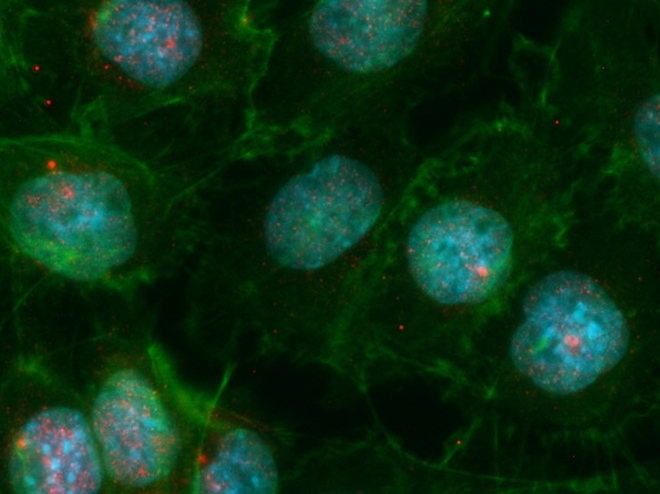
Brazilian researchers use an editing tool to investigate a gene that plays a key role in eliminating autoaggressive cells and controlling the development of diseases such as type 1 diabetes (image: immunofluorescence image of mTECs, with cell nucleus highlighted in blue and Aire genes shown as red dots / Karina F. Bombonato-Prado, FORP-USP)
By Elton Alisson | Agência FAPESP – The human immune system sometimes fails to recognize tissue and organs as healthy body parts and attacks them as if they were harmful invaders. This identification error is called aggressive autoimmunity and can lead to diseases such as autoimmune polyglandular syndrome type 1 (APS-1) and type 1 diabetes.
Scientists have discovered in recent years that two genes active in medullary thymic epithelial cells (mTECs) control aggressive autoimmunity: Fezf2 (forebrain-expressed zinc finger 2) and, above all, Aire (autoimmune regulator).
A group of researchers at the University of São Paulo (USP) in Brazil, affiliated with its Ribeirão Preto Medical School (FMRP) and Ribeirão Preto Dental School (FORP), used a DNA editing tool called CRISPR/Cas9 to manipulate Aire in order to understand better how this gene controls autoimmune diseases.
The study, which resulted from a research project supported by FAPESP and from the master’s research conducted by Cesar Augusto Speck-Hernandez at FMRP-USP, was published in the journal Frontiers in Immunology.
“For the first time, we used CRISPR/Cas9 to block Aire in cultured murine mTECs and to study the effect of loss of this gene’s function,” said Geraldo Aleixo Passos, a professor at FMRP-USP and FORP-USP and the principal investigator for the project.
As Passos explained to Agência FAPESP, autoimmune disease is triggered by autoantibodies (antibodies directed against the organism) or by autoaggressive T lymphocytes. These cells, which originate in thymocytes, are “educated” in the thymus (a gland located just behind the sternum in the middle of the chest) not to attack elements of their own organism.
When this education fails, the thymus allows autoaggressive T lymphocytes to escape into the body, and they may attack organs such as the adrenal or suprarenal glands (causing APS-1) or the pancreas, where they destroy insulin-producing cells and cause the development of type 1 diabetes.
Researchers in the field of immunology always associate the function of Aire with the elimination of autoaggressive thymocytes, since APS-1 patients, for example, have mutations in this gene’s DNA sequence, but until now, the link had never been irrefutably demonstrated.
“We decided to test the hypothesis that Aire is involved in eliminating autoaggressive thymocytes by controlling their physical adhesion or contact with mTECs. In the absence of physical contact with mTECs, autoaggressive thymocytes aren’t eliminated,” Passos said.
Editing the gene
The researchers suspected that if Aire mutations are found in autoimmune disease patients, this must mean the gene has lost its function of controlling adhesion between mTECs and autoaggressive thymocytes.
They tested this hypothesis by using CRISPR/Cas9 to disrupt the DNA of Aire in murine mTECs and cause mutations in the gene that made it lose its original function.
A gene must be complete and lacking in deleterious mutations in order to work properly. When its DNA is disrupted using CRISPR/Cas9, the cell activates an emergency “repair” system to splice the two strands back together again before it dies. Because this repair system is not perfectly efficient, the cell itself may make mistakes in sequencing the target gene, and the result is a mutation.
“The mutant target gene usually loses its original function, and this causes some kind of problem in the mutant cell,” Passos explained.
The researchers found that Aire-mutant mTECs were worse at adhering to thymocytes than their normal (wild-type) counterparts.
When they sequenced the transcriptomes of the Aire-mutant and wild-type mTECs, they observed that Aire also controls messenger RNA sequences (mRNAs) that encode the proteins involved in cell-cell adhesion. The transcriptome is the complete set of RNA molecules in a cell, from protein-coding mRNAs to noncoding RNAs.
In a previous study conducted as part of the master’s research of Nicole Pezzi, supervised by Passos, the researchers used a gene silencing technique called RNA interference to show that Aire controls adhesion between mTECs and thymocytes.
“These new findings reinforce the theory that Aire is involved in adhesion between mTECs and thymocytes, a key process for the elimination of autoaggressive cells and prevention of autoimmune disease,” said Passos, who is affiliated with the Center for Research on Inflammatory Diseases (CRID), one of the Research, Innovation and Dissemination Centers (RIDCs) funded by FAPESP.
In his view, the use of CRISPR/Cas9 opens up important new research prospects in editing the genome of laboratory mouse mTECs to mimic Aire mutations found in autoimmune disease patients.
“This will greatly facilitate research into the effects of pathogenic Aire mutations,” Passos said. “The human and murine genomes are very similar in terms of DNA sequences [over 80% identical], so we can continue using CRISPR/Cas9 on mouse cells to study the mechanisms of aggressive autoimmunity in humans and, in future, maybe try to control them.”
The Frontiers in Immunology article “Aire disruption influences the medullary thymic epithelial cell transcriptome and interaction with thymocytes” (doi: 10.3389/fimmu.2018.00964) by Cesar A. Speck-Hernandez et al. can be read at frontiersin.org/articles/10.3389/fimmu.2018.00964/full.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





