

Results published in PNAS pave the way for the development of inhibitors that could be tested in parasites (image: release)
Results published in PNAS pave the way for the development of inhibitors that could be tested in parasites.
Results published in PNAS pave the way for the development of inhibitors that could be tested in parasites.
Results published in PNAS pave the way for the development of inhibitors that could be tested in parasites (image: release)
By Karina Toledo | Agência FAPESP – In an article published in the journal Proceedings of the National Academy of Sciences (PNAS), researchers affiliated with the University of São Paulo (USP) in Brazil describe the structure of an enzyme that plays a key role in the metabolism of Leishmania major, the protozoan that causes cutaneous leishmaniasis.
According to the authors, the results of their research, which was supported by FAPESP, offer new perspectives for the development of drugs that could be useful to treat the various types of leishmaniasis as well as Chagas disease and sleeping sickness (African trypanosomiasis).
“The protein we found in L. major is very similar to the protein in Trypanosoma cruzi, which causes Chagas, and in T. brucei, which causes sleeping sickness. On the other hand, it’s very different from the enzyme found in humans. That makes it a highly interesting target for drug discovery,” said Maria Cristina Nonato, a professor at the University of São Paulo’s Ribeirão Preto School of Pharmaceutical Sciences (FCFRP-USP) and principal investigator for the study.
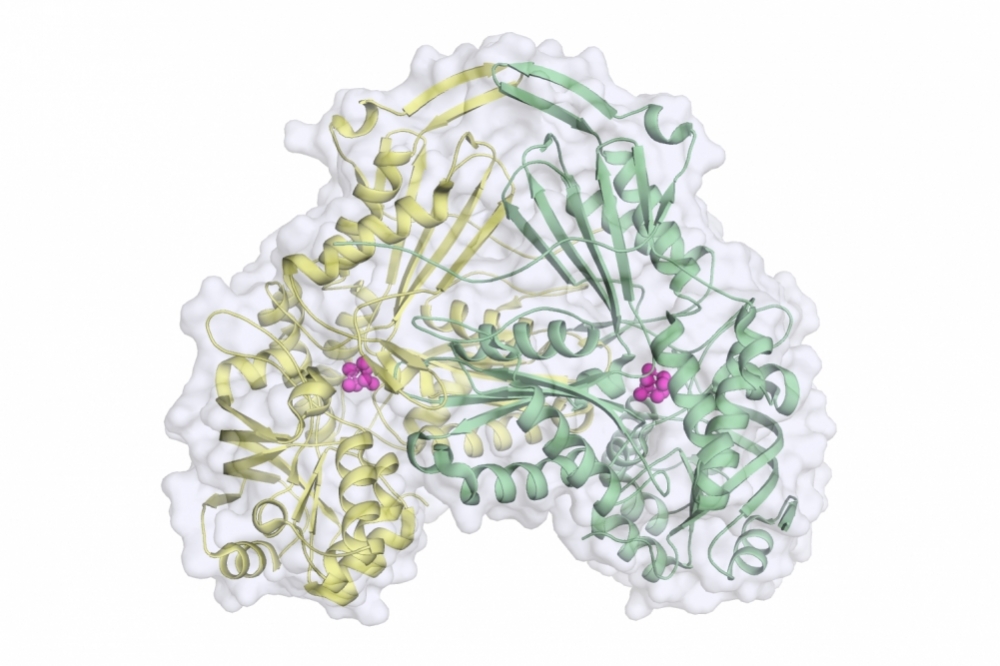
Known as fumarate hydratase, the protein described in the article is responsible for catalyzing the reversible hydration/dehydration of fumarate to malate. Both metabolites are important to parasite survival.
In humans, fumarate hydratase deficiency is associated with several pathologies, such as fumaric aciduria, considered an inborn error of metabolism and characterized by weak muscle tone, psychomotor retardation and convulsions, among other symptoms, as well as cutaneous and uterine leiomyomatosis (benign smooth muscle tumors) and renal cell carcinoma.
In the article, the researchers show that when this protein folds, it assumes a three-dimensional shape that resembles an upside-down heart and that was previously undescribed in the scientific literature. Their studies also identified the main amino acid residues involved in the catalytic reaction. This knowledge, according to Nonato, is a potential basis for the development of enzyme activity inhibitors that could be tested to evaluate their efficacy as anti-parasite medications.
Sensitive to oxygen
The work began during Patricia Rosa Feliciano’s research for her master’s degree and PhD, which Nonato supervised. This was when the protein cloning, bacterial expression and purification methods were developed.
However, progress was only possible thanks to a partnership with Catherine Drennan, a researcher at the Massachusetts Institute of Technology (MIT) in the US, because the activity of fumarate hydratase is inhibited by contact with oxygen. As a result, it cannot be properly studied without a glove box, a sealed container into which nitrogen is pumped to remove all the oxygen and create an inert atmosphere, accessible only via gloves.
“Brazil doesn’t yet have an ideal infrastructure for working with air-sensitive proteins,” Nonato said. “The purification and crystallization for structure determination were done at MIT in partnership with Drennan and with the help of a FAPESP scholarship for a research internship abroad.”
With the protein purified and crystallized, the group investigated its three-dimensional structure using X-ray diffraction crystallography.
“By measuring how the protein crystal diffracts a beam of X-rays, we can discover the type of atom present and its position in the molecule,” Nonato explained. “The correlation between the atomic content and the diffraction pattern is used to reconstruct the protein’s structure. The crystal contains many copies of the same molecule, which amplifies the signal.”
Next, the group created mutant versions of the protein and also investigated these versions’ crystal structure and mapped their catalytic mechanism. The findings from this second part of the research project will be published shortly.
“We’ve actually developed an inhibitor, and it works well, but as yet we don’t have the results of its effects on parasites,” Nonato said.
In previous studies, the group showed that two isoforms of fumarate hydratase are found in L. major: one in mitochondria and another in the cytosol, the fluid portion of the cytoplasm, which contains the cell’s water-soluble components. According to Nonato, the mitochondrial isoform is involved in producing energy for the parasite.
“What’s most interesting is that these are two very similar proteins, yet they play different roles in the organism, so when you inhibit them, you interfere with more than one metabolic pathway,” Nonato said. “We therefore believe the molecule can have a stronger effect than drugs that interfere with only one pathway.”
Leishmaniasis is transmitted by the bite of an infected sand fly (genus Lutzomyia). The cutaneous form presents with skin ulcers, while the mucocutaneous form also presents with ulcers of the mucosa of the upper respiratory tract.
In Brazil, the disease is mostly caused by the protozoan L. braziliensis. Nonato performed the study with L. major because the species is the first to have had its genome sequenced.
Other protozoans of the same genus cause visceral leishmaniasis, considered one of the six most important parasitic diseases affecting humans.
The article “Crystal structure of an Fe-S cluster-containing fumarate hydratase enzyme from Leishmania major reveals a unique protein fold” (doi: 10.1073/pnas.1605031113) can be retrieved from pnas.org/content/113/35/9804.abstract.
More images and information about the enzyme fumarate hydratase in L. major can be viewed on the website of the Protein Crystallography Laboratory: lcprp.com.br/paginas/index_en.php.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





