
Project funded in the so-called SMOLBnet 2.0 studies the family of protein kinases in the search for inhibitors to combat certain types of cancer and neglected diseases
Project funded in the so-called SMOLBnet 2.0 studies the family of protein kinases in the search for inhibitors to combat certain types of cancer and neglected diseases.
Project funded in the so-called SMOLBnet 2.0 studies the family of protein kinases in the search for inhibitors to combat certain types of cancer and neglected diseases.

Project funded in the so-called SMOLBnet 2.0 studies the family of protein kinases in the search for inhibitors to combat certain types of cancer and neglected diseases
By Mônica Pileggi
Agência FAPESP – The key to the development of medications to combat certain types of cancer and neglected tropical diseases (such as Chagas Disease and Leishmaniasis) could be in the molecular and structural study of a family of protein kinases called Nek, involved in the progression of the cellular cycle.
The study entitled “SMOLBNET 2.0: Functional and structural studies of protein kinases involved in cancer and neglected diseases: towards the development of new inhibitors,” selected in the call for proposals of the Network of Structural Biology in Advanced Life Sciences Topics-- SMOLBnet 2.0, studies the atomic interactions between potential inhibitors and the Nek kinases to develop medications able to stop oncogenesis and the advance of illnesses caused by parasites like Trypanossoma cruzi and Leishmania ssp.
The goal of the SMOLBnet network, a FAPESP program, is to promote partnerships between research groups that have experience in resolution of macromolecule structure through crystallography with X rays or nuclear magnetic resonance (NMR) and research groups from the molecular area that develop competitive, high impact projects in complex biological systems.
The initial phase of the project, coordinated by Jörg Kobarg, an accredited professor in Functional and Molecular Biology and in Molecular Genetics and Biology from the Universidade Estadual de Campinas (Unicamp) and researcher at the National Biosciences Laboratory (LNBio) involved the characterization of the interactoma—network of interactions between cellular components—of Nek proteins.
Not largely explored, Neks are the focus of the research. Studies showed that these kinases have, in some types of cancer like gastric cancer, altered expressions or activities.
The Nek6—one of the 11 proteins in the Nek family identified in humans—especially calls attention. According to Kobarg, the attraction of this kinase is its capacity to interact with many proteins (some 60 were identified) forming a network in which it sits at the center.
“Usually, a protein interacts with just a few proteins in the cell to carry out its function. But because it plays a central role in the network of interactions, the Nek6 is a promising target for inhibition,” the scientist told Agência FAPESP.
The functional interaction with the remaining proteins happens by way of a process called phosphorylation. Here, the Nek6 adds a phosphate group (PO4) to another protein, altering its function. “This is a fundamental event, as it acts like a molecular circuit breaker. The substrate protein is oftentimes activated by phosphorylation,” he explained.
When the kinase protein is activated or expressed in an excessive manner, it can deregulate the cell, causing it to grow in a disorderly manner, a common characteristic of carcinogenic cells. “By inhibiting the Nek6 activity, we hope to provoke cellular death or interrupt the advance of the protein during the cellular cycle so that we can stop cancer from developing,” pointed out Kobarg.
In the search for potential inhibitors, large-scale triage bioassays are performed where it is possible to observe the enzymatic activity of the kinase in a series of compounds of diverse origins (commercial, synthetic and natural products).
Selective inhibitors
Based on the biological function study of the kinases and human Nek family carried out by Kobarg and his colleagues, the group coordinated by Tyago Murakami, accredited professor in the post-graduate programs in Functional and Molecular Biology and Molecular Genetics and Biology at Unicamp as well as LNBio researcher, studies the activity of similar proteins in Trypanossoma cruzi and Leishmania ssp, as potential targets for the development of medications.
“The objective is to integrate and exchange knowledge that we need to understand the biological role of these enzymes and reveal their three-dimensional structures. The information generated are essential for understanding the biology of the parasite and for developing new therapies,” highlighted Murakami.
“The idea of the study is to identify selective inhibitors that should act on the parasite only. This is a challenge, because the medication used to eliminate the parasite could also have collateral effects on humans,” he said.
The scientist explains that living beings, in general, have orthologous molecules, meaning they have the same functions as the organism. This means that proteins considered essential for the survival of the parasite could have similar function in the human body.
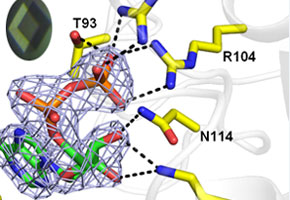
In order to attempt to elucidate the inhibition mechanism of compounds in the kinases of the protozoa, aside from deciphering the protein’s structure (both the human and the parasite) the following techniques are used: single-crystal X ray diffraction, fluorescence spectroscopy, circular dichroism, calorimetry, magnetic resonance imaging and small-angle X-ray scattering (SAXS). "Kinases are challenging proteins, as they are very flexible and modular. This makes it hard to get crystals,” he said.
In the same phase of the study, Murakami and his colleagues also made a sweep with a library of specific kinase inhibitors. This process allowed them to identify promising molecules, able to selectively block the Leishmaniasis parasite’s protein while maintaining the function of human ortholgy.
“Based on these data, today we work with a diphosphate nucleoside kinase from Leishmania. Aside from this, we obtained the structure of this protein, which allowed us to begin mapping the active site of this kinase and perform comparative analyses with the similar human protein,” he explained.
This kinase is considered to be important for the parasite’s invasion process. When it is secreted by the protozoa, it intermediates the interactions between the host and the parasite, regulating the level of ATP in the host cell and prolonging its half-life. “The inhibition of this protein has the potential to attenuate the dissemination and development of the parasite in the human body,” he said.
Even though they are preliminary data, the good news is also related to the search for inhibitors for the T. Cruzi protozoa. According to Murakami, both the Chagas Disease parasite and the Leishmaniasis parasite show similar inhibition profiles. “When we use the screening strategy, both show the same behavior,” he said.
The next step of the research, explained Murakami, is to carry out in vivo tests to understand the function of the inhibitor molecule in physiological conditions. This phase will take place in partnership with the Oswaldo Cruz Foundation (Fiocruz).
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





