

Animation shows the vibrating molecules and the bond between the two iodine molecules stretching nearly 50% – from 0.27 to 0.39 millionths of a millimeter – before returning to its initial state (image: release)
The method that allows observation of the chemical bonds between atoms on a scale of one millionth of one billionth of a second has applications in a wide variety of fields.
The method that allows observation of the chemical bonds between atoms on a scale of one millionth of one billionth of a second has applications in a wide variety of fields.
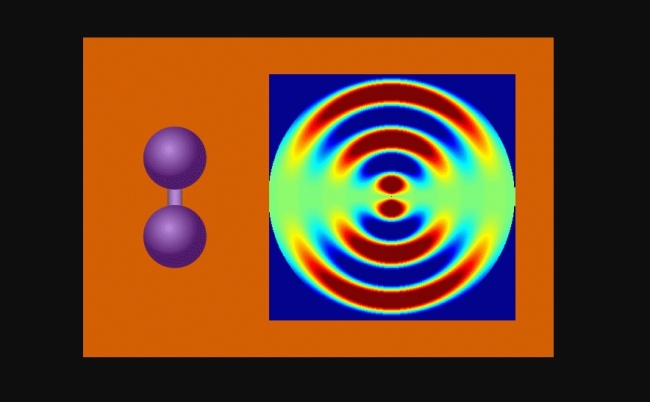
Animation shows the vibrating molecules and the bond between the two iodine molecules stretching nearly 50% – from 0.27 to 0.39 millionths of a millimeter – before returning to its initial state (image: release)
By Heitor Shimizu, in Lincoln (USA) | Agência FAPESP – The chemical bonds between atoms are flexible. That flexibility is what allows molecules to change shape in essential processes for chemical and biological functions, such as breathing in humans or photosynthesis in plants.
They are movements that occur on a time scale known as a femtosecond. One femtosecond, or one quadrillionth of a second (10-15) is the time it takes for light to travel the distance equivalent to the thickness of a human hair. Until now, studies of chemical bonds between atoms have been indirect, such as spectroscopy, which requires calculations to transform data into images of nuclear geometry.
The group led by Martin Centurion, a professor in the Department of Physics and Astronomy at the University of Nebraska-Lincoln, in the United States, has developed a method to directly observe continuous movements at the nuclear level.
Using an ultrafast electron camera assembled in partnership with researchers from the SLAC National Accelerator Laboratory, at Stanford, Centurion and his colleagues were able, for the first time, to record direct images from the nucleus of an atom in which the molecules are vibrating within millionths of a billionth of a second after being hit by a laser pulse.
“We were able to capture vibrations with atomic resolution,” said Centurion during FAPESP Week Nebraska-Texas, which is bringing together researchers from the United States and Brazil through September 22 in the cities of Lincoln (Nebraska) and Lubbock (Texas).
The method they used in the video, known as ultrafast electron diffraction (UED), could help scientists better understand the role nuclear movements play in processes directed by light that naturally occur in extremely fast time scales.
“Our idea was to understand and control the conversion of light into other forms of energy at the molecular level. Light is converted into mechanical energy (vibration) or into chemical energy by the breaking of bonds and the changes in the shape of the molecule studied. We wanted to observe a chemical reaction at the time it occurs, at the atomic level and in ultrafast time scales,” he said.
The researchers were able to observe iodine molecules at various points after they were stimulated by a laser pulse. When gathering the images, they created an animation that shows the vibrating molecules and the bond between the two iodine molecules stretching nearly 50% – from 0.27 to 0.39 millionths of a millimeter – before returning to its initial state. Each registered vibrational cycle lasted nearly 400 femtoseconds.
The concept behind the method is similar to a classic physics experiment – conducted first by British physicist Thomas Young in 1801 – in which a laser beam passes through a pair of vertical slits, producing a pattern of bright and dark areas on a screen. The pattern formed depends on the distance between the two slits.
In UED, an electron beam shines through a gas of iodine molecules. The distance between the two iodine nuclei in each molecule defines the double slit. “First the laser excites the molecules. Then the electron pulse disperses them. The molecular structure is obtained from the diffraction of the electrons, forming a diffraction pattern,” Centurion said.
The laser passes through the slits and hits a detector on a screen. The resulting pattern is the diffraction pattern referred to by the researcher. “The distance between the slits can be calculated from the frequency of the interference pattern” Centurion said.
As the iodine molecules vibrate, the pattern changes. These changes in the separation of the nuclei were recorded to make the animation that shows the molecular flexibility.
The researcher’s group was also successful in experiments that generated highly charged laser pulses, with more than one billion electrons per pulse, more than 1,000 times faster than traditional methods.
“Laser accelerated electron pulses are promising ways for capturing the interaction of matter with intense laser fields in which the image needs to be captured in a single shot,” he said.
Centurion emphasizes that ultrafast electron diffraction will create new opportunities for conducting high precision studies of dynamic processes in several fields such as biology, chemistry and materials science.
The UED method has been studied by groups of researchers in various countries since the 1980s, but the quality of the electron beam has only recently become good enough to allow studies on the scale of the femtosecond.
Centurion and his colleagues at SLAC used a piece of equipment that has an ultrabright high energy electron source, originally developed for the laboratory’s x-ray laser known as the Linac Coherent Light Source.
The researchers now plan to use the method to study larger and more complex molecules than iodine. The findings of the study by Centurion and his colleagues, “Diffractive Imaging of Coherent Nuclear Motion in Isolated Molecules”, were published in the journal Physical Review Letters.
More information about FAPESP Week Nebraska-Texas is available at: fapesp.br/week2017/nebraska-texas.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





