
Antiviral potential of FDA-approved drugs tested; five compounds selected as most promising (image: release)
Antiviral potential of FDA-approved drugs tested; five compounds selected as most promising.
Antiviral potential of FDA-approved drugs tested; five compounds selected as most promising.

Antiviral potential of FDA-approved drugs tested; five compounds selected as most promising (image: release)
By Karina Toledo | Agência FAPESP – To find new weapons with which to combat infection by Zika virus, a group of Brazilian and French researchers have tested the efficacy of 725 compounds approved for human use by the US Food & Drug Administration (FDA) but with a range of different indications.
Five compounds were selected as the most promising, including the antiemetic palonosetron. Results of the research project, which is supported by FAPESP, have been published via the online open science platform F1000Research.
“This is just the first step in the development of drugs against Zika,” said Lucio Freitas Junior, a researcher at Butantan Institute in São Paulo, Brazil, and principal investigator for the project. “Based on this result, we can use medicinal chemistry tools to modify the structures of these compounds and make their antiviral action even more powerful. On the other hand, recently discovered molecules can be tested immediately in animal models or even clinical trials of efficacy against Zika, because they’ve already been approved as drugs for human use. It’s a way of reducing lead times and research costs for new drug development.”
This strategy, known as drug repositioning, has been used by Freitas Junior’s group for several years in a search for treatments for neglected tropical diseases. Freitas Junior was previously a researcher at the National Energy & Materials Research Center (CNPEM).
The latest study was part of the postdoctoral research performed by Bruno dos Santos Pascoalino with a scholarship from FAPESP and with the collaboration of Gilles Courtemanche, currently Antimicrobials Unit Director at Bioaster Technology Research Institute in Paris, France, and formerly with Sanofi, a French pharmaceutical company.
“Courtemanche has worked in the pharmaceutical industry for more than 20 years, and thanks to his expertise, we were able to select the drugs with the most suitable characteristics in terms of distribution and metabolism,” Freitas Junior said. “Among the reasons why palonosetron was considered the most promising compound were its high bioavailability and its ability to cross the blood-brain barrier, which protects the central nervous system against potentially toxic substances present in the bloodstream – very important in the case of Zika, a virus that’s strongly attracted to nerve tissue.”
Methodology
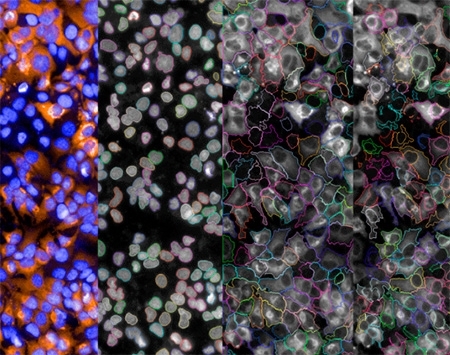
The group used a high-content screening (HCS) methodology to sift through a library of 725 FDA-approved drugs with the aim of identifying compounds with selective activity against Zika virus in human cells. The Zika virus used in the assays was isolated from a patient in Recife, Pernambuco State, during the 2015 epidemic.
The model was based on Huh7 human hepatoma (liver) cells similar to those used in the discovery and development of new drugs against hepatitis C. Zika virus and hepatitis C have something in common: both belong to the virus family Flaviviridae. However, Zika belongs to the genus Flavivirus, while hepatitis C belongs to the genus Hepacivirus.
The antiviral potential of the screened compounds was compared with that of interferon α-2A (IFNα2A), a human protein that is produced by immune cells and is highly active in vitro against several viruses, including Zika.
“Image analysis enabled us to determine the percentage of cells infected in each case. We developed a computer program that automatically selected the substances with behavior that most closely resembled that of the control, IFNα2A,” Freitas Junior said.
A total of 29 compounds were found to display some activity against Zika, he added, but the group prioritized those with the most potential for use in treating infection by the virus, according to the criteria used by the pharmaceutical industry.
Among those selected were lovastatin, used to treat hypercholesterolemia; 5-fluorouracil, a chemotherapy drug used in cancer treatment; 6-azauridine, an antimetabolite known to inhibit the replication of viral RNA; kitasamycin, a macrolide antibiotic with a broad spectrum of antibacterial activity; and palonosetron, a serotonin receptor antagonist used in the treatment of chemotherapy-induced nausea and vomiting.
According to Freitas Junior, palonosetron is also widely prescribed for morning sickness during pregnancy, although its safety profile for newborns is currently controversial.
“Because these five drugs are or have been marketed, their pharmacokinetics and pharmacodynamics are fairly well known,” Freitas Junior said. “With this information, it’s easier and faster to design dosing and administration protocols for tests in animal models or even in humans.”
The group is now working with collaborators in Brazil and abroad, he added, to create other versions of the selected molecules. “This will enable us to increase their antiviral activity tenfold or more,” he said. “Some Brazilian researchers already have analogues of palonosetron and have contacted us to start tests against Zika. We also plan to collaborate with the pharmaceutical companies that work with this class of molecule.”
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





