
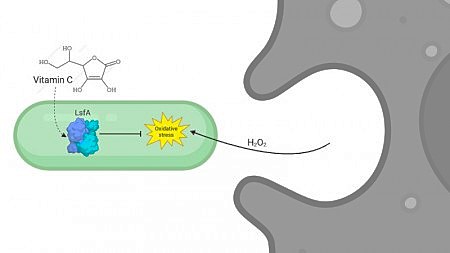
Interaction allows the enzyme to regenerate after oxidation, restoring its antioxidant function (image: Rogério Aleixo/BioRender)
Findings contribute to the search for new targets against hospital infections.
Findings contribute to the search for new targets against hospital infections.

Interaction allows the enzyme to regenerate after oxidation, restoring its antioxidant function (image: Rogério Aleixo/BioRender)
Agência FAPESP* – Throughout evolution, pathogenic microorganisms, such as bacteria, viruses, and fungi, have developed sophisticated defense strategies to survive and multiply in the hostile environment of their hosts. These mechanisms increase their virulence and make infections more difficult to fight. One of the most effective strategies is the neutralization of oxidants released by defense cells to eliminate invaders.
A research group led by Luis Eduardo Soares Netto, from the Institute of Biosciences at the University of São Paulo (IB-USP) and the Center for Redox Processes in Biomedicine (Redoxoma), describes how the LsfA protein (a type 1-Cys peroxiredoxin) protects Pseudomonas aeruginosa bacteria against hydrogen peroxide produced during the immune response. LsfA catalyzes the elimination of hydroperoxides using ascorbate (vitamin C) as a reducing agent, thereby strengthening the bacteria's antioxidant defense.
Redoxoma is a Research, Innovation, and Dissemination Center (RIDC) funded by FAPESP and based at USP's Institute of Chemistry (IQ).
"A major contribution of our work is the structural determination of a protein involved in the virulence of a medically important bacterium. We also demonstrated that ascorbate can act as a reducing agent in a cellular system, which is something new. In technical terms, we're the first to use the HyPer7 probe in Pseudomonas," said Rogério Luis Aleixo Silva, a researcher at the University of Massachusetts Chan Medical School in the United States and a former FAPESP scholarship holder. Silva participated in the research as a doctoral student at IB-USP.
The unprecedented structural data obtained in the study may open up new opportunities for developing specific inhibitors of the bacterial enzyme and advancing new therapeutic strategies.
"This is the first study to characterize the biochemistry and structure of a bacterial Prx6. In the literature, among the three major domains of life – Eubacteria, Archaea, and Eukarya – there are already many resolved structures of Prx6, mainly in Archaea and mammals, including humans. But this is the first structure of a bacterial Prx6. E. coli, which is a classic model bacterium, doesn't have Prx6," comments Netto.
The research results were published in an article in the journal Redox Biology.
Antioxidant system
Pseudomonas aeruginosa is an opportunistic bacterium that primarily causes infections in people with weakened immune systems. It causes various types of hospital-acquired infections, including pneumonia in patients with cystic fibrosis, urinary tract infections, and infections in burns and surgical wounds. It can also cause endocarditis and septicemia. Due to its resistance to antibiotics, it is one of the priority bacterial pathogens for the development of new treatments on the World Health Organization (WHO) list.
When the body is infected by a pathogen, it reacts by mobilizing immune defenses such as phagocytes. These cells fight microorganisms by releasing reactive oxygen, nitrogen, and chlorine species. Faced with this oxidative stress, bacteria such as P. aeruginosa activate protective mechanisms involving various antioxidant proteins, including peroxiredoxins (Prxs).
LsfA, a peroxiredoxin of the Prx6 subfamily, is present in P. aeruginosa and is associated with bacterial virulence. In the new study, the researchers deepened their understanding of the molecular mechanisms involved in its protective function. They showed although P. aeruginosa has an arsenal of antioxidant enzymes, LsfA stands out for its high efficiency in decomposing hydrogen peroxide.
A key finding of the study is the interaction between LsfA and ascorbate. In 2007, Netto's group showed that this vitamin can reduce sulfenic acid, which is formed during the oxidation of 1-Cys peroxiredoxins. This finding challenges the prevailing view that these enzymes depend exclusively on thiol recycling. A thiol is an organic compound containing a sulfur atom bonded to a hydrogen atom, analogous to the sulfur in alcohols. Thiols play important roles as antioxidants, helping to protect cells.
While the role of vitamin C as a Prx reducer remains unclear in biological systems, structural analyses from the study suggest that ascorbate interacts directly with the active site of LsfA. This interaction enables the enzyme to regenerate after oxidation and restore its antioxidant function.
Inhibitor
Since bacterial LsfA has a human homolog, any potential inhibitor should target only the bacterial form without affecting the human form. The researchers demonstrated that, despite its structural similarity to other Prx6 proteins, bacterial LsfA exhibits unique electrostatic properties, resulting in distinct charges at its active sites. These differences influence how an inhibitor interacts with each version of the enzyme.
"The advantage of our study is that, in addition to solving the structure, we used in silico docking to show some of the interactions between LsfA and ascorbate that could perhaps be mimicked by an inhibitor," says Aleixo-Silva.
According to Netto, the next steps include investigating ascorbate metabolism in P. aeruginosa further, as well as conducting studies using macrophage models to assess the effects of LsfA exclusion on bacterial defense and host inflammatory responses.
The article "Interaction between 1-Cys peroxiredoxin and ascorbate in the response to H2O2 exposure in Pseudomonas aeruginosa" can be read at www.sciencedirect.com/science/article/pii/S2213231725001715.
* With information from Maria Celia Wider from Redoxoma.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





