

Image viewed under fluorescence microscope shows nuclei of retinal cells (to left of arrow) and ciliary epithelial cells (to right of arrow) in mice (image: Dânia Emi Hamassaki)
Scientists suggest strategies that stimulate cells in ciliary epithelium to proliferate and return to progenitor state.
Scientists suggest strategies that stimulate cells in ciliary epithelium to proliferate and return to progenitor state.
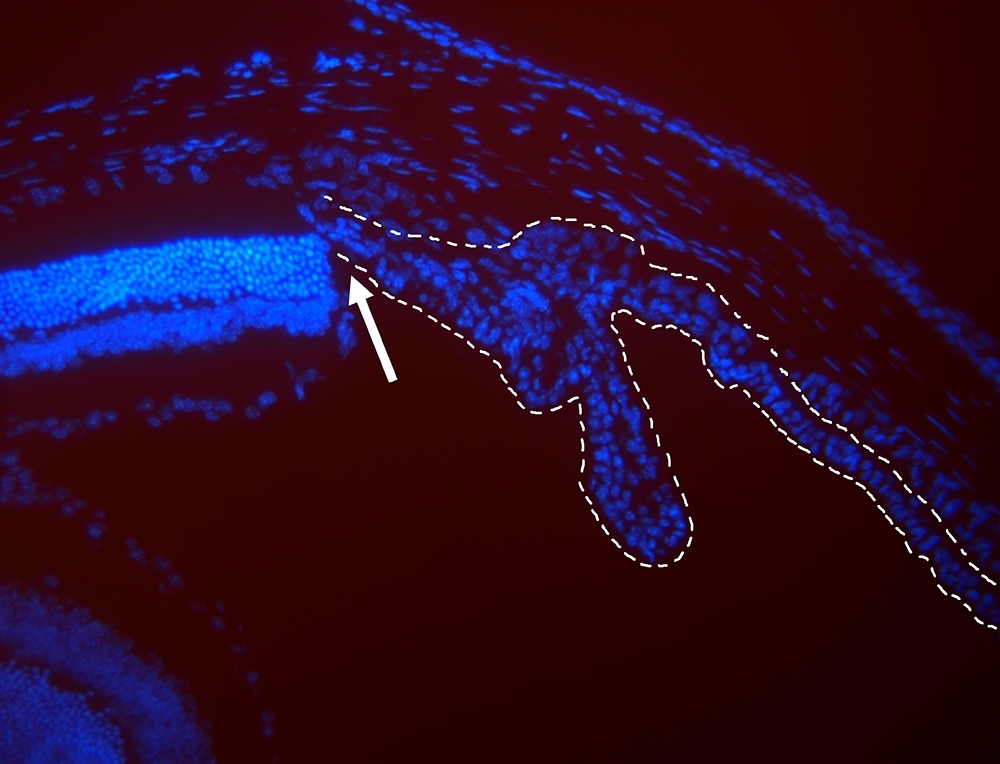
Image viewed under fluorescence microscope shows nuclei of retinal cells (to left of arrow) and ciliary epithelial cells (to right of arrow) in mice (image: Dânia Emi Hamassaki)
By Elton Alisson
Agência FAPESP – A structure in the human eye called the ciliary body, located between the retina and the root of the iris, is responsible for lens accommodation and for producing aqueous humor, the colorless fluid that fills the chambers of the eye and maintains intraocular pressure.
The ciliary body is lined with a type of tissue called the ciliary epithelium, which is formed by two layers of cells.
Approximately 15 years ago, scientists discovered that this structure also contains quiescent (inactive) cells, which, under specific conditions, can be stimulated to become stem or progenitor cells capable of proliferating and differentiating into types of cells lost to retinal degeneration.
A study performed by researchers at the Department of Cell & Development Biology, part of the University of São Paulo’s Biomedical Science Institute (ICB-USP), now points to possible ways of stimulating the proliferation or reprogramming of these ciliary epithelial cells in mice.
The study was conducted as part of a project supported by FAPESP. Its findings were published in the journal Investigative Ophthalmology & Visual Science.
“We observed that stimulating one of the ciliary epithelial cell signaling pathways in a certain manner leads to an increase in the proliferation or progenitor profile of these cells,” Dânia Emi Hamassaki, a professor at ICB-USP and the principal investigator on the project, told Agência FAPESP.
The researchers activated and inactivated signaling proteins belonging to the Rho GTPase family in the ciliary epithelium of mice.
This family of proteins regulates multiple signaling pathways that control gene transcription, cell survival and proliferation, note the authors of the study.
To evaluate the effect of these proteins on cell cycle regulation and ciliary epithelium progenitor cell expression, the researchers expressed and activated the GTPases RhoA, RhoB and Rac1 in mouse ciliary epithelial cells by intraocular injection of lysophosphatidic acid (LPA), a bioactive lipid that plays an important role in cell proliferation, migration and death.
In another experiment, they inhibited the proteins with toxin A of Clostridium difficile, a bacterium that is naturally present in the intestinal flora of some humans.
The results of the experiments showed that inhibition of GTPases caused retinal progenitor cells to proliferate in the ciliary epithelium.
Activation of GTPases by LPA induced an increase in retinal progenitor cells in the ciliary epithelium but did not influence proliferation of this cell type in this region of the eye.
“We found that modulation of these two different mechanisms – proliferation and reprogramming of ciliary epithelial cells – may provide a potential new approach to retinal tissue repair,” Hamassaki said.
Limited regeneration
According to the researcher, ciliary epithelial cells, as well as the Müller glial cells found in the retina itself, have been identified as potential therapeutic targets in light of their contribution to neurogenesis (formation of new retinal neurons) in adults, given their stem and progenitor cell properties.
Although it is a relatively conserved biological mechanism, the capacity for regeneration of neurons lost after retinal injuries – which occur in cases of glaucoma, diabetic retinopathy and macular degeneration, for example – varies across different species of animals, Hamassaki explained.
“In the course of evolution, animals have lost the capacity to regenerate retinal neurons, which is very well established in fish and amphibians and much more limited in birds and mammals,” she said.
One hypothesis that might explain the progressive loss of retinal neuron regeneration, according to Hamassaki, is the existence of some inhibitory mechanism that prevents cells in the retina itself (glial cells) or in the ciliary epithelium of some animals, for example, from proliferating and differentiating into neurons.
Postdoctoral researcher Carolina Beltrame Del Debbio is conducting a project to identify such a mechanism with a scholarship from FAPESP and under Hamassaki’s supervision.
A better understanding of this inhibitory mechanism and of how retinal neurons die and can be repaired may enable ciliary epithelial and glial cells to be manipulated so that they can proliferate and differentiate into cell types that have been lost within the retina, Hamassaki explained.
“This retinal or epithelial cell reprogramming strategy, such that the cells performing the functions of cell types lost to retinal degeneration, is very interesting because they’re already in the right microenvironment,” she said.
The article “Rho GTPases control ciliary epithelium cells proliferation and progenitor profile induction in vivo” (doi: 10.1167/iovs.13-13162), by Hamassaki et al., can be read in the journal Investigative Ophthalmology & Visual Science at www.iovs.org/content/55/4/2631.abstract.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





