

The hormone was administered directly into the brains of obese mice (image: American Journal of Physiology – Endocrinology and Metabolism)
An experiment conducted at the State University of Campinas showed that FGF19, produced in the intestine, acts on specific regions of the brain, causing the body to burn energy to produce heat; the discovery paves the way for new drugs.
An experiment conducted at the State University of Campinas showed that FGF19, produced in the intestine, acts on specific regions of the brain, causing the body to burn energy to produce heat; the discovery paves the way for new drugs.
The hormone was administered directly into the brains of obese mice (image: American Journal of Physiology – Endocrinology and Metabolism)
By Luciana Constantino | Agência FAPESP – Research carried out on mice has revealed how a hormone released by the intestine acts on the brain and helps regulate the body’s energy expenditure. FGF19 (fibroblast growth factor 19) activates mechanisms that stimulate the use of more energy, burn fat, and favor weight control and blood glucose levels in obese animals.
These effects were associated with the action of FGF19 in the hypothalamus, a specific brain region that integrates peripheral and environmental signals to regulate energy metabolism. The authors of the study identified that FGF19 signaling in the hypothalamus leads to increased thermogenic adipocyte activity (i.e., fat cells that burn energy to produce heat).
The discovery could contribute to the development of new lines of drugs against obesity, diabetes, and other metabolic disorders based on substances that mimic the action of endogenous compounds, i.e. those produced by the body itself.
This approach is similar to some cutting-edge diabetes therapies currently on the market for patients with obesity. One example is Ozempic, whose active ingredient, semaglutide, activates receptors that mimic the hormone GLP-1 and signal satiety to the brain.
According to the study, FGF19 also reduced peripheral inflammation and promoted cold tolerance. However, these benefits disappeared when the activity of the sympathetic nervous system was inhibited in an experiment. The scientists found that exposure to cold increased the expression of FGF19 receptors in the hypothalamus. The hypothalamus plays an important role in maintaining body temperature, suggesting an adaptive role for FGF19 in energy balance and thermoregulation.
“FGF19 had already been linked to a reduction in food intake. Our work broke new ground by showing that it also plays an important role by acting on the hypothalamus and stimulating an increase in energy expenditure in white and brown adipose tissue. In other words, in addition to controlling appetite, it stimulates thermogenesis. So, in terms of therapy associated with obesity, it’d make a lot of sense,” explains Professor Helena Cristina de Lima Barbosa, from the Obesity and Comorbidities Research Center (OCRC) at the State University of Campinas (UNICAMP).
The OCRC is a Research, Innovation, and Dissemination Center (RIDC) of FAPESP, which also supported the study through grants (22/15148-2 and 20/14020-7) awarded to doctoral student Lucas Zangerolamo, first author of the work, under Barbosa’s guidance.
The article describing these findings has been published in the American Journal of Physiology – Endocrinology and Metabolism and was highlighted by the journal as a Top Article in May.
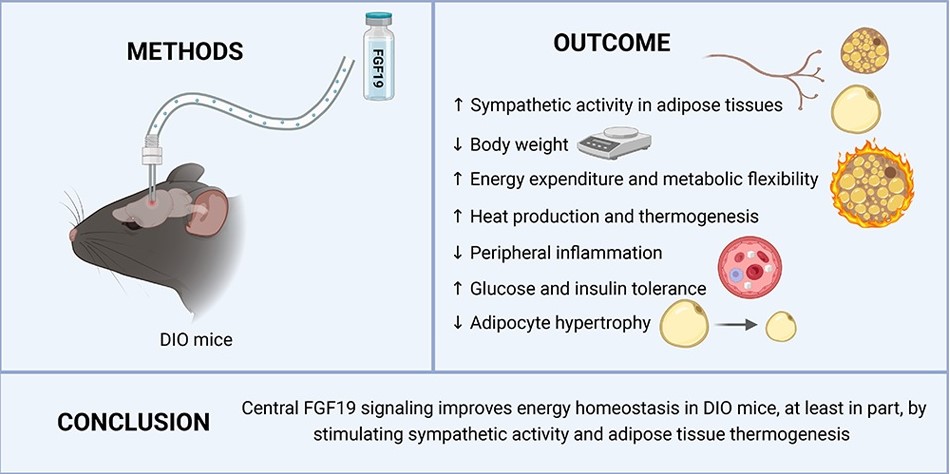
A graphic summary of the study (image: American Journal of Physiology – Endocrinology and Metabolism)
Scenario
The World Atlas of Obesity 2025 points out that based on current trends, the world will not achieve this year’s targets for preventing and controlling chronic non-communicable diseases. These goals include stopping the increase in diabetes and obesity, as well as reducing premature mortality from cardiovascular, chronic respiratory, and cancer diseases by 25%, using 2010 data as a baseline.
According to the Atlas, over 1 billion people worldwide live with obesity. Projections indicate that this number could exceed 1.5 billion by 2030 if effective measures are not implemented. Obesity is linked to 1.6 million premature deaths per year caused by non-communicable diseases.
In Brazil, approximately 31% of people are obese, and between 40% and 50% of adults do not exercise at the recommended frequency or intensity.
Step-by-step research
FGF19, which is involved in the control of energy metabolism, is mainly produced in the small intestine. In the liver, it regulates the production of bile acids, as well as the synthesis of glucose and fats. While its primary functions in the liver have been well-documented in scientific literature, its signaling in the brain has received limited analysis.
“In the lab, we work with bile acids, which are also the subject of my master’s degree, and they regulate the release of FGF-19. Our initial studies led us down this path,” Zangerolamo told Agência FAPESP.
At eight weeks of age, the animals were randomly divided into two groups: one with a regular diet (control) and one with a high-fat diet. The hormone was administered directly into the brains of the obese mice. The animals were housed in an environment controlled for temperature, lighting, and water supply.
In the article, the scientists point out that central FGF19 signaling improved energy homeostasis by increasing sympathetic nervous system activity and stimulating adipose tissue thermogenesis, causing the tissue to burn more energy in the form of heat.
“The brain plays an extremely important role in controlling the body’s adiposity. At the same time as it receives information from peripheral tissues, it triggers commands. These commands, apparently using the sympathetic nervous system, seem to be an interesting way of thinking about energy expenditure,” adds Barbosa.
The authors compiled and analyzed public scRNA-seq data from different papers on the hypothalamus. This technique allows the RNA of individual cells to be sequenced. The authors analyzed the transcription of more than 50,000 single cells to identify hypothalamic cell types that express FGF19 receptors.
The researchers explain that a key issue now is understanding how to stimulate the body to produce more FGF19. The group continues to work to understand how the pathways involved in eating behavior are linked to this process.
“We want to broaden this understanding. We’re studying the hypothalamus to evaluate the inflammation commonly observed when a high-fat diet is administered and whether FGF19 plays a role in this area,” says Zangerolamo, who did part of the work during an internship at the Joslin Diabetes Center at Harvard Medical School with Professor Yu-Hua Tseng, who is also an author of the article.
The article “Central FGF19 signaling enhances energy homeostasis and adipose tissue thermogenesis through sympathetic activation in obese mice” can be read at: journals.physiology.org/doi/full/10.1152/ajpendo.00488.2024.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





