

In an article published by Scientific Reports, researchers characterize a subgroup of neurons in the olfactory epithelium that express CD36, a lipid metabolism regulator (photo: release)
In an article published by Scientific Reports, researchers characterize a subgroup of neurons in the olfactory epithelium that express CD36, a lipid metabolism regulator.
In an article published by Scientific Reports, researchers characterize a subgroup of neurons in the olfactory epithelium that express CD36, a lipid metabolism regulator.
In an article published by Scientific Reports, researchers characterize a subgroup of neurons in the olfactory epithelium that express CD36, a lipid metabolism regulator (photo: release)
By Karina Toledo | Agência FAPESP – A paper by Brazilian researchers published in the journal Scientific Reports describes a study showing that a subgroup of olfactory neurons in the nasal cavity express a cellular receptor specializing in the transport of lipid molecules.
Known as CD36, this membrane receptor is usually intensely expressed in fatty tissue, where it regulates lipid metabolism. It also plays a well-known role in the immune system, where it is required for phagocytosis of potentially harmful molecules by macrophages. Previous research has linked the presence of CD36 in the tongues of mammals with a preference for high-fat food.
Experiments with Drosophila fruit flies have identified the existence of a homologous gene to CD36 called SNMP, which participates in antenna signaling through the pheromone cVA (11-cis-vaccenyl acetate) to mediate mating responses and aggressiveness between individuals of the same species.
“The existence of CD36 in nasal sensory neurons is a novel discovery and appears to point to olfactory detection of lipids in mammals. Like the tongue, this may be linked to a preference for fatty food,” said Isaias Glezer, a professor in the Biochemistry Department at the Federal University of São Paulo (UNIFESP).
“Another possibility is that this receptor is linked to perception of smells by individuals of the same species and to social interactions, since there is evidence of the existence of lipidic pheromones.”
Glezer is principal investigator for the FAPESP-funded research project entitled “Post-lesion cell regeneration in the nervous system and functional aspects of genes linked to innate immune response”.
The discovery was made in a study of the nasal mucous membrane in mice, in collaboration with Bettina Malnic at the University of São Paulo’s Chemistry Institute (IQ-USP) and Fabio Papes at the University of Campinas’s Biology Institute (IB-UNICAMP), as well as with support from the Center for Research on Redox Processes in Biomedicine (Redoxoma), one of the Research, Innovation and Dissemination Centers (RIDCs) funded by FAPESP.
The researchers now plan to confirm whether the lipid molecule receptor is also present in human olfactory epithelium. According to Glezer, the initial aim of the investigation was to understand the role played in neurons by CD36 and other molecules associated with innate immune response.
“In a sort of lucky dip, we set out to see whether this receptor was expressed in the olfactory epithelium,” he said. “We were surprised to find it intensely expressed in certain groups of neurons found throughout the nasal cavity, but not in all. This was a totally unexplored field.”
To investigate the function of these olfactory neurons that express CD36, they performed experiments with two groups of mice, one wild-type group (not genetically modified) and another group comprising mice of the same lineage that did not express CD36.
The researchers measured the times taken by each of the groups to explore a piece of paper soaked in saline solution and another piece of paper scented with a mixture of fatty acids. The wild-type animals (with CD36) spent far more time exploring the lipid-scented paper than the saline-soaked paper, while in the group without CD36 the difference was not significant.
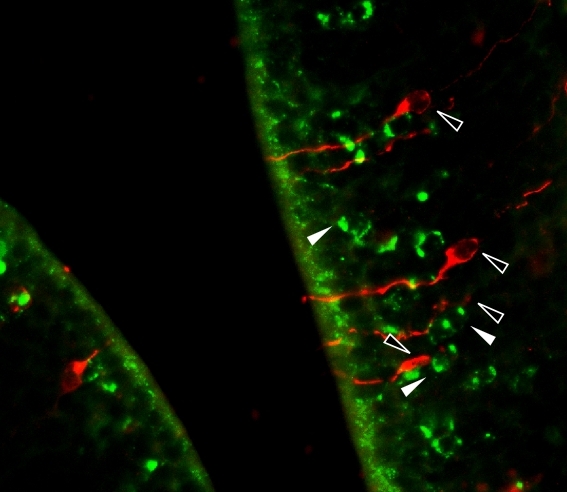
“On its own, of course, this doesn’t prove that CD36 receptors in these sensory neurons are responsible for olfactory detection of lipids and the preference for this almost imperceptible smell, but it’s strong evidence in that direction,” Glezer said. “In addition, we showed that CD36 concentrates in the ciliary layer of the olfactory epithelium, the portion responsible for odorant detection. However, more evidence was required for the purposes of scientific journal peer review.”
Subpopulation of sensory neurons
Glezer and his collaborators therefore decided to investigate whether CD36 expression in the olfactory epithelium was linked to the expression of any other olfactory receptors. There are approximately a thousand different genes that encode olfactory receptors in mice, according to Glezer, and each olfactory neuron expresses only one of these genes.
“We analyzed the neurons that expressed the most abundant olfactory receptors in the tissue and found no correlations with CD36 in any of them,” he said. “We then requested the assistance of a UK research group who were studying the gene expression profiles of olfactory neurons one by one using single-cell RNA sequencing. We asked them to see if they could identify a neuron that expressed CD36.”
The UK group in question was led by Darren Logan at the Wellcome Trust Sanger Institute. Thanks to this collaboration, PhD student André Machado Xavier, lead author of the article, was able to demonstrate a correlation between CD36 expression and expression of a gene that encodes an olfactory receptor called Olfr287.
“So, we can state that there is indeed a subpopulation of neurons which express CD36 and Olfr287 concomitantly. This is no accident. It must have a function, and more research is required to discover what that function is,” Glezer said.
Among the research group’s future plans is a project to explore in greater detail the functioning of the Olfr287 receptor in order to discover how it is activated and what happens to it in the absence of CD36, for example.
“At Redoxoma, we’re studying mice that don’t express CD36 to see if there are any alterations associated with the constitution of their olfactory epithelium and its oxidation of lipids,” Glezer said.
The article entitled “CD36 is expressed in a defined subpopulation of neurons in the olfactory epithelium” (doi:10.1038/srep25507) by André Machado Xavier et al. can be read at www.nature.com/srep/2016/160505/srep25507/full/srep25507.html.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





