

Microscopy images showing differences in retinal vascular thickness for mice (clockwise): without retinopathy, with induced retinopathy treated with peptide, with retinopathy treated with placebo, with untreated retinopathy (image: Science Advances)
Molecule described in Science Advances inhibited pathological growth of new blood vessels in preclinical trials.
Molecule described in Science Advances inhibited pathological growth of new blood vessels in preclinical trials.
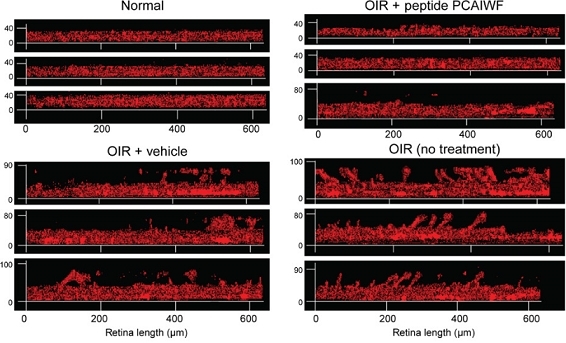
Microscopy images showing differences in retinal vascular thickness for mice (clockwise): without retinopathy, with induced retinopathy treated with peptide, with retinopathy treated with placebo, with untreated retinopathy (image: Science Advances)
By Karina Toledo | Agência FAPESP – A small synthetic peptide identified by researchers at the University of São Paulo’s Chemistry Institute (IQ-USP) in Brazil has the potential to inhibit the pathological growth of new blood vessels that occurs in retinopathy and cancer, according to a report on preclinical trials.
The trials were performed during Jussara Michaloski Souza’s postdoctoral research, with Professor Ricardo Jose Giordano as principal investigator. The project was supported by FAPESP, and its results have recently been published in the journal Science Advances.
“The peptide isn’t a drug yet, but it can serve as a model for the development of a new angiogenesis inhibitor,” Giordano said in an interview given to Agência FAPESP.
Angiogenesis is the growth of blood vessels from existing vasculature, he explained. It can occur physiologically as part of wound healing or when demand for oxygen and nutrients increases in a particular tissue, but it is often pathological.
In the case of diabetic retinopathy, for example, excessive blood sugar leads to abnormally abundant and disorganized retinal blood vessel growth, damaging the tissue and potentially impairing eyesight. In some types of cancer, tumors release mediators that induce intense angiogenesis to increase the flow of oxygen and nutrients so that uncontrolled proliferation of malignant cells can continue.
The main mediators involved in angiogenesis are four proteins that belong to the vascular endothelial growth factor (VEGF) family. Known as VEGF A, B, C and D, they bind to specific receptors on the cell surface (proteins VEGFR 1, 2 and 3), triggering a cascade of intracellular signaling to start the formation of new blood vessels.
“The peptide we describe in the paper, with the amino acid sequence PCAIWF, proved capable of binding to all three VEGF receptors on the cell surface and blocking the action of the entire family of proteins,” Giordano said.
Discovery
To find the molecule that interacted most effectively with the extracellular portion of the receptors, Giordano’s group developed and screened a library of almost 10 billion different peptides using a technology called phage display.
Phage display is an in vitro screening technique for the study of protein-protein, protein-peptide and protein-DNA interactions by manipulating the genomes of bacteriophages (viruses that infect bacteria) so that each viral particle synthesizes a different peptide, which binds to its surface protein.
“We used bacteriophages because they’re viruses that are highly resistant to changes in temperature and pH, so the peptide library we created will remain viable for research for years,” Giordano explained.
The next step was to incubate the entire library with one VEGFR at a time in order to see which viral particles would bind to which receptor. “Initially, we focused only on VEGFR 3, which until then was the least studied in connection with angiogenesis. The idea was to identify a peptide that would bind to this receptor and find out what would happen if it was blocked,” he said.
The first round of assays pointed to the peptide PCAIWF as the most promising. When the researchers conducted new in vitro assays with the purified molecule (no longer coupled to the bacteriophage), they discovered that it also bound to VEGFR 1 and 2, blocking the action of the entire VEGF family.
“Each of the proteins binds to different receptors,” Giordano said. “VEGF A, for example, binds to VEGFR 1 and 2, but not to VEGFR 3. VEGF C binds to VEGFR 2 and 3, but not to VEGFR 1. So by blocking all three receptors, we inhibited the action of all proteins in this class, which suggests more effective action.”
In vivo trials
To test the effect in vivo, the researchers used a murine model that simulates retinopathy of prematurity. In human babies, this condition is caused by overexposure to oxygen in a neonatal incubator. The gas inhibits the formation of blood vessels in the retina, which normally occurs in the last few weeks of gestation. When a baby leaves the incubator, the ocular tissue suffers from hypoxia (lack of oxygen), and pathological angiogenesis occurs.
“Mouse pups are born with their eyes closed and don’t open them for about two weeks,” Giordano said. “Retinal blood vessels only begin to form after they’re born, and we can simulate the pathological process that occurs in premature babies.”
Seven-day-old mouse pups were placed in an oxygen chamber until they were 12 days old. On the 15th day after birth, some were given an intraocular injection with the peptide PCAIWF. Their retinas were examined two days later, when angiogenesis should have peaked.
Signs of retinopathy were evident in the animals treated with placebo, whereas total vascular area and depth of vasculature in the retinas of the group treated with the peptide were similar to those of the group that had not been placed in the oxygen chamber and therefore displayed normal retinal development.
Next steps
According to Giordano, the results obtained during Souza’s postdoctoral research opened up new lines of investigation. One of these, on which work has already begun, involves an in-depth study of the peptide’s structure using methods such as nuclear magnetic resonance (NMR) to understand how it interacts with receptors.
“This knowledge can be the basis for rational design of new angiogenesis inhibitors, possibly to be administered orally,” Giordano said. “However, although the peptide isn’t ideal as a drug because it would have to be injected directly into the eye, which is unpleasant, it may be possible to develop slow-release nanoformulations so that injections could be spaced out.”
An injectable biopharmaceutical called bevacizumab currently available on the market acts by neutralizing the action of VEGF A. “This is a monoclonal antibody that neutralizes the action of the most important protein for the process of angiogenesis,” Giordano explained. “It’s been used to treat colon and kidney cancer and gliomas as well as retinopathy. But it’s expensive. Each dose costs about R$5,000, and monthly injections are required.”
Another option is sunitinib, a drug that offers the advantage of being orally administered but has more adverse side effects because its action is systemic. “It acts on the intracellular portion of the VEGF receptors and ends up also affecting other similar proteins, such as the platelet-derived growth factor or PDGF receptor,” Giordano said. “Without the action of PDGF, blood vessels are more fragile and hemorrhagic, and this can affect the heart above all.”
As for PCAIWF, he explained, the peptide acts on the extracellular portion of the VEGF receptors, where these proteins are most different from others in the same class (tyrosine kinases). Nevertheless, the risk of adverse side effects is far from negligible if it is orally administered, he believes, since it could affect the physiological process of angiogenesis in other tissues.
“At present, we’re working with animal models to try to identify genes expressed only in pathological angiogenesis, which could be an avenue to the development of even more selective drugs,” Giordano said.
The article “Discovery of pan-VEGF inhibitory peptides directed to the extracellular ligand-binding domains of the VEGF receptors” (doi: 10.1126/sciadv.1600611) can be read at advances.sciencemag.org/content/2/10/e1600611.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





