

Research group studies functions of proteins involved in calcium signaling in Trypanosoma cruzi and helps to identify drug development targets (image: Noelia Lander)
Research group studies functions of proteins involved in calcium signaling in Trypanosoma cruzi and helps to identify drug development targets.
Research group studies functions of proteins involved in calcium signaling in Trypanosoma cruzi and helps to identify drug development targets.
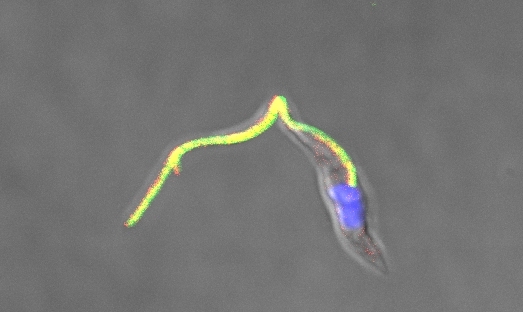
Research group studies functions of proteins involved in calcium signaling in Trypanosoma cruzi and helps to identify drug development targets (image: Noelia Lander)
By Karina Toledo | Agência FAPESP – Scientists have frequently used techniques to manipulate the genomes of disease-causing parasites in an endeavor to identify targets for drug development. These techniques include inhibiting or stimulating the expression of proteins to investigate their functions.
While progress has been achieved in the study of Trypanosoma brucei, which causes sleeping sickness, and Toxoplasma gondii, which causes toxoplasmosis, in the case of Trypanosoma cruzi, which causes Chagas disease, the methods traditionally used to knock out genes or tag intracellular proteins have thus far been ineffective.
With support from FAPESP through the Thematic Project “Calcium signaling in trypanosomatids”, a group led by Roberto Docampo at the University of Campinas’s Medical School (FCM-UNICAMP) in São Paulo State, Brazil, has shown that the genome of T. cruzi can be manipulated using a system called CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 (CRISPR-associated gene 9).
Docampo is a professor at the University of Georgia in the United States and, for over 25 years, has collaborated with Aníbal Vercesi, a professor at UNICAMP. Together, they discovered a novel organelle called the acidocalcisome, which is involved in the regulation of calcium levels in cells, in T. cruzi. Docampo is the principal investigator for the Thematic Project under the aegis of a São Paulo Excellence Chair Program developed by Vercesi’s laboratory at UNICAMP.
The CRISPR/Cas9 methodology consists of transfecting the parasite by deliberately introducing nucleic acids containing a vector – in this case, a small circular DNA molecule called a plasmid – into its cells. This induces the expression of two other molecules, Cas9 and single guide RNA (sgRNA), which merge in cell nuclei to form a ribonucleoprotein. Cas9 is an endonuclease, an enzyme that breaks down double-stranded DNA into two or more shorter chains. The sgRNA serves to guide the enzyme to the target gene so that genetic material can be introduced into the site of the DNA break or suppressed from it. In addition to the plasmid, a donor DNA molecule is transfected to repair the double-stranded DNA.
“The system was first described as a rudimentary immune system in bacteria. Later, it was adapted for genome editing in eukaryotic organisms. Today, it can be used in practically any kind of organism,” said Noelia Lander, a postdoctoral fellow at FCM-UNICAMP.
A study by the same group, published in the journal mBio in 2015, showed for the first time that the CRISPR/Cas9 system can be used to knock out genes in T. cruzi.
More recently, in an article published in The Journal of Biological Chemistry (JBC), the group also validated the technology as a method of protein tagging, a process used to locate proteins inside cells and identify signaling pathways that are important to the parasite’s survival.
“We performed what is known as C-terminal protein tagging, adding to the polypeptide chain a small molecule such as hemagglutinin, against which specific antibodies are commercially available,” Lander explained. “The antibodies recognize the molecule and bind to it. We can then locate the protein we’re studying in the cell using fluorescence microscopy.”
To confirm that the technique worked in T. cruzi, the group first used the CRISPR/Cas9 system to tag the protein TcVP1 (vacuolar proton pyrophosphatase), whose localization was already known.
“TcVP1 is present in the acidocalcisome. Specific antibodies against it exist, and they pointed to the same localization as the one we observed when we used the CRISPR/Cas9 method,” Lander said.
Next, the CRISPR/Cas9 system was used to tag and localize the genes encoding three other proteins: TcFCaBP (flagellar calcium-binding protein), found in the parasite’s flagellum; TcMCU (mitochondrial calcium uniporter), identified in its mitochondria; and TcIP3R (inositol-1,4,5-triphosphate receptor), whose localization in the acidocalcisome was confirmed.
“With regard to TcIP3R, the localization was disputed in the literature: a Japanese research group led by Katsuhiko Mikoshiba defended the theory that it was in the endoplasmic reticulum, whereas Docampo said it was in the acidocalcisome, which we succeeded in confirming,” Lander said.
Ramifications
The discovery not only clears up a longstanding dispute, Lander added, but also has other ramifications, such as facilitating a better understanding of the cellular signaling mechanisms mediated by calcium in the parasite.
“Calcium signaling is important to the parasite’s survival and contributes to its capacity to infect the host,” Lander said. “Calcium signals have been described as important to the process of host cell invasion.”
Currently, the group is using the CRISPR/Cas9 system to study the function of TcMCU and TcIP3R with the aim of confirming whether the calcium signaling pathway can be adopted as a target for therapy against T. cruzi.
“What we’re doing basically is knocking out the gene that encodes the protein or inducing its overexpression. We then characterize the phenotypes of these mutant lineages,” Lander said.
Some of the experiments in the Thematic Project are being performed as part of Lander’s postdoctoral research and that of Miguel Angel Chiurillo Siervo, with support in both cases being provided by FAPESP.
“Our aim in studying the function of several proteins involved in calcium signaling is to identify an enzyme or metabolic route that’s essential to the parasite but not present in humans. We can then validate it as a therapeutic target,” Lander said.
The article “CRISPR/Cas9-induced disruption of paraflagellar rod protein 1 and 2 genes in Trypanosoma cruzi reveals their role in flagellar attachment” can be read at mbio.asm.org/content/6/4/e01012-15.abstract.
To access the article “CRISPR/Cas9-mediated endogenous C-terminal tagging of Trypanosoma cruzi genes reveals the acidocalcisome localization of the inositol-1,4,5-trisphosphate receptor,” click here.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





