

Inflammation caused by uric acid does not depend on high plasma levels of the substance or a mechanical lesion due to uric acid crystals, according to scientists at the Center for Research on Redox Processes in Biomedicine (image: release)
Inflammation caused by uric acid does not depend on high plasma levels of the substance or a mechanical lesion due to uric acid crystals, according to scientists.
Inflammation caused by uric acid does not depend on high plasma levels of the substance or a mechanical lesion due to uric acid crystals, according to scientists.
Inflammation caused by uric acid does not depend on high plasma levels of the substance or a mechanical lesion due to uric acid crystals, according to scientists at the Center for Research on Redox Processes in Biomedicine (image: release)
By Maria Fernanda Ziegler | Agência FAPESP – Everything happens very quickly, in a matter of milliseconds. The chemical reactions between uric acid and enzymes in the bloodstream play a key role in triggering the inflammatory processes that cause serious health problems, such as kidney stones, gout, and cardiovascular diseases like atherosclerosis.
Even plasma uric acid levels considered normal can unleash a reaction that is harmful to tissue. This discovery was made by scientists at the Center for Research on Redox Processes in Biomedicine (Redoxome), one of the Research, Innovation and Dissemination Centers (RIDCs) funded by FAPESP.
They studied the chemical mechanism whereby uric acid is transformed in the organism and how it reacts with proteins. The results of their study, which identified the main targets of the uric acid reaction, were published in an article in The Journal of Biological Chemistry.
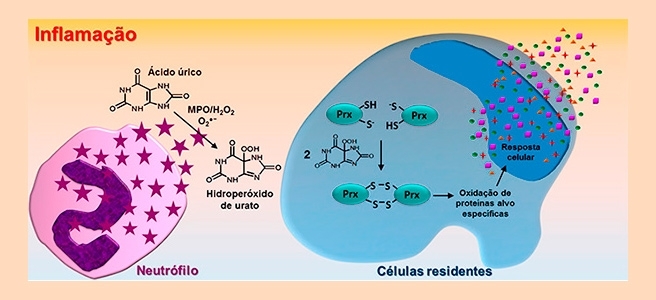
When uric acid accumulates in the bloodstream, it is known to cause crystals to form in joints, resulting in profound inflammation of the surrounding tissue. The researchers at Redoxome succeeded in proving that blood vessels can be damaged even if crystal formation does not occur.
“The damage caused by uric acid is silent. Even if it doesn’t cause gout, it can be metabolized by enzymes, the heme peroxidases, producing highly reactive intermediates. These intermediates are uric acid free radicals and urate hydroperoxide,” said Flávia Carla Meotti, a professor in the Biochemistry Department of the University of São Paulo’s Chemistry Institute (IQ-USP) in Brazil.
Urate hydroperoxide is a key compound for vascular inflammation. The researchers showed that this compound reacts rapidly and preferentially with abundant blood cell proteins called peroxiredoxins.
“To identify the proteins most likely to react with urate hydroperoxide, our group calculated how long it took for a reaction to occur between urate hydroperoxide and these proteins,” Meotti said.
Oxidation of peroxiredoxins by urate hydroperoxide can affect cell functions. “The reaction between peroxiredoxins and urate hydroperoxide may alter the pattern of expression of other proteins and make cells more likely to release pro-inflammatory mediators, fueling a vicious circle of inflammatory response,” Meotti said.
The production of urate hydroperoxide by inflammatory processes has never been demonstrated before, Meotti added. “During inflammation, cells produce a number of oxidizing compounds to combat invasive microorganisms,” she said. “Formation of the oxidant urate hydroperoxide under these same conditions is a new discovery that has been investigated by our group for eight years in collaboration with researchers in New Zealand.”
This line of research could lead to findings that help in the diagnosis of vascular lesions and even in the identification of therapeutic targets for use in preventing cardiovascular disease.
“We do basic research,” Meotti said. “Our discoveries can be used to identify a new factor that helps predict the risk of developing atherosclerosis and put forward new ways of preventing this condition.”
Paradoxical effects of uric acid
Uric acid forms when the nucleic acids DNA and RNA are broken down. During evolution, humans ceased to express the enzyme that breaks down uric acid, which is why it accumulates in the bloodstream.
“This evolutionary trait has always been considered an advantage because uric acid is an anti-oxidant and can donate electrons, combating free radicals and other oxidizing substances,” Meotti said. “On the other hand, when it donates just one electron from its valence layer, which is the reaction that occurs with heme peroxidases, uric acid itself becomes a free radical.
“The combination of this free radical with superoxide forms urate hydroperoxide. Paradoxically, given that uric acid is a powerful anti-oxidant, uric acid free radicals and urate hydroperoxide are both powerful oxidants.”
The article “Urate hydroperoxide oxidizes human peroxiredoxin 1 and peroxiredoxin 2” (doi: 10.1074/jbc.M116.767657), by Larissa A. C. Carvalho, Daniela R. Truzzi, Thamiris S. Fallani, Simone V. Alves, Jose Carlos Toledo Junior, Ohara Augusto, Luis E. S. Netto and Flavia C. Meotti, can be read at jbc.org/content/early/2017/03/27/jbc.M116.767657.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





