

A technique based on composite ceramic membranes promises to be more efficient than those currently available. The study was performed by scientists in Brazil and Portugal (image: release)
A technique based on composite ceramic membranes promises to be more efficient than those currently available. The study was performed by scientists in Brazil and Portugal.
A technique based on composite ceramic membranes promises to be more efficient than those currently available. The study was performed by scientists in Brazil and Portugal.
A technique based on composite ceramic membranes promises to be more efficient than those currently available. The study was performed by scientists in Brazil and Portugal (image: release)
By Elton Alisson | Agência FAPESP – The search for new technologies to capture and store carbon dioxide (CO2) has intensified along with the growing concern to decrease the impact of this greenhouse gas on global warming.
A technology recently developed by researchers at the Materials Science & Technology Center of Brazil’s Energy & Nuclear Research Institute (IPEN), in collaboration with colleagues at the University of Aveiro in Portugal, is based on composite ceramic membranes. A composite is a combination of two or more materials yielding properties beyond those of the individual components. This technology promises to be more efficient than the methods currently available to separate CO2 from gas mixtures.
The project is being conducted under the aegis of the Center for Research and Development of Functional Materials (CDFM), one of the Research, Innovation and Dissemination Centers (RIDCs) supported by FAPESP.
“Essentially, what we’ve tried to develop is a system that can separate CO2 from gas mixtures like factory smoke containing CO2 as a pollutant, for example, or the mixture emitted when a fuel like natural gas is burned,” said Fernando Manuel Bico Marques, a professor in the University of Aveiro’s Materials & Ceramics Engineering Department (UA-DEMAC) and one of the researchers involved in the project, in an interview given to Agência FAPESP.
According to Marques, who is in Brazil to develop part of the project at IPEN with FAPESP’s support, the available CO2 separation technologies are based on the use of absorption solvents, solid adsorbents as separators, and membranes.
In absorption, the molecules of a fluid mix with those of a solid or liquid. Adsorption occurs when molecules adhere to the surface of a solid or liquid.
Among these technologies, electrochemical separation membranes have taken the lead as the most promising solution mainly because they consume less energy and are scalable.
However, today’s separation membranes, which consist of inorganic materials, organic materials such as polymers, or a combination of these two classes of materials, cannot fully separate out CO2 from gas mixtures or operate at temperatures higher than about 400 °C.
“It isn’t a trivial task to separate CO2 from a mixture such as that produced by burning natural gas, which can reach 1,000 °C and, besides CO2, contains carbon monoxide, sulfur oxide, water vapor and several other molecules of different sizes,” Marques said.
“For this reason, separation membrane systems must be highly selective and capable of operating at quite high temperatures.”
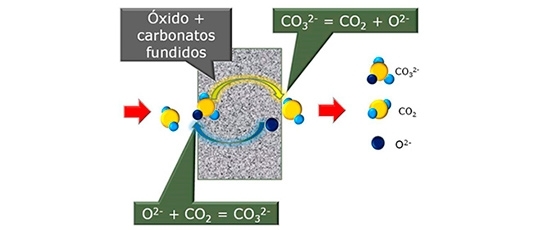
To overcome these barriers, the researchers plan to develop composite membranes for selective CO2 separation at high temperatures by combining electrolytes (solutions that allow the passage of electrons) in cerium oxide fuel cells with fused alkali carbonate.
In this electrochemical system, the cerium oxide-based electrolyte acts as a conductor of oxide ions, and the alkali carbonate acts as a conductor of carbonate ions.
When the CO2 molecules in a gas mixture are passed through a ceramic membrane, they combine with oxide ions to form CO32− (carbonate ions).
The carbonate ions are transported by the fused alkali carbonate across the membrane to the opposite side, where they decompose and release CO2.
The oxide ions then return to their original positions in the membrane, where they combine with CO2 molecules and resume the process of separating the compound from gas mixtures.
“The main challenge in producing high-temperature selective CO2 separation membranes is how to combine these two materials, cerium oxide and fused alkali carbonate, in a composite microstructure capable of guaranteeing that the system has maximum efficiency,” Marques said.
Industrial applications
According to Marques, the group, having demonstrated the concept of using composite membranes for selective CO2 separation, next plans to enhance the system’s efficiency to a level at which it will becomes promising for industrial applications.
“In the field of membranes, the benchmark for a highly efficient CO2 separation system is 1 milliliter per square centimeter per minute. So far, we’ve reached about 60% of that level,” he said.
The cost of this technology may be highly competitive with existing systems, because it uses well-known and widely used materials (solid oxide and fused carbonate), Marques noted.
Carbonates are cheap, and only a small amount of cerium oxide is used. “Besides the low cost of the materials involved, the system can operate for many years without requiring maintenance,” he said.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





