
Research work of FAPESP Scientific Initiation fellow on platinum oxide structures is published in Physical Review B, published by American Physical Society
Research work of FAPESP Scientific Initiation fellow on platinum oxide structures is published in Physical Review B, published by American Physical Society.
Research work of FAPESP Scientific Initiation fellow on platinum oxide structures is published in Physical Review B, published by American Physical Society.

Research work of FAPESP Scientific Initiation fellow on platinum oxide structures is published in Physical Review B, published by American Physical Society
By Elton Alisson
Agência FAPESP – Ricardo Kita Nomiyama, a fourth year graduate student in chemistry at the Universidade de São Paulo, São Carlos campus, has managed to do what few of his peers achieve in their academic careers.
Nomiyama’s scientific initiation research has just been published in the on-line edition of Physical Review B, a publication of the American Physical Society, in one of the prime sections of the publication. Entitled “Structural properties of oxides using the theory of density functional theory,” the study was conducted with FAPESP funding.
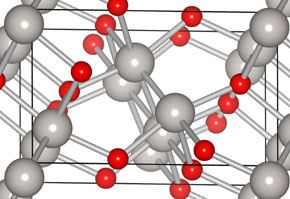
During his research, conducted during the third year of graduate studies, Nomiyama utilized a computational tool – known as the density functional theory implemented in VASP code – to study the atomic structure of platinum oxide.
Through his research, he discovered a monoxide platinum structure with lower energy than the other proposed some 70 years ago by Nobel Laureate in Chemistry, Linus Pauling (1901-1994), in addition to two atomic structures of platinum dioxide that are as stable as the previous discoveries.
“With the knowledge that Nomiyama had in his third year of graduate studies and aided by a computational tool, he was equipped to make a very relevant scientific contribution on a problem that has been studied for years,” comments researcher Juarez Lopes Ferreira da Silva, who was his project mentor, in an interview with Agência FAPESP.
Silva is pursuing his post-doctorate at the Physics Institute of USP- São Carlos and is conducting research "Computational catalysis: production of hydrogen using ethanol", funded through Young Investigators in Emerging Centers (JP-FAPESP).
Silva requested that Nomiyama calculate energy in conjunction with the roughly 20 platinum monoxide structures that were described by Pauling in a study published in a 1941 edition of Journal of American Chemical Society.
He also oriented the student to look for new platinum monoxide structures that could also have low energy based on a set of new structures found in other experiments. To this end, Nomiyama suggested looking for structures in a stage of oxidation similar or near that of platinum oxide, and made calculations from simulations on existing materials. In comparing them with candidate structures, he discovered that they had lower energy than the others.
Intrigued with the discovery, Silva and a doctoral candidate from his research group repeated all the calculations made by Nomiyama to verify whether he had really found a new structure or if he had committed an error. They verified that the result he had obtained was correct. “He really found a structure that has lower energy than the others that had been proposed 70 years ago. And the energy is the most important fact for determining the stability of a system,” explains Silva.
The next step was to extend the study to other compositions like platinum dioxide. In this stage of the experiment, however, Nomiyama did not managed to find a lower energy structure, but found two others with energy as good as the existing structures.
Contributions
According to Silva, the results of the study are important for advancing knowledge on the structure of platinum. “Taking into consideration that platinum is one of the most widely used elements in catalysis (increasing the speed of a chemical reaction) and that oxygen is present in almost every chemical reaction that occurs in nature, now we have information about oxide that is present in the surface of the majority of materials that form platinum,” he explains.
Platinum is the main material utilized in the anodes (electrodes) of ethanol fuel cells to break the ethanol molecule and obtain hydrogen to inject into the fuel cell. Because during this reaction species formation occurs with a large quantity of oxygen – which could connect to the platinum surface, diminishing the effect of the catalyzer – the USP research group focused on understanding oxygen’s interaction with platinum.
“Understanding this interaction is fundamental for advancing the development of low-cost, high stability catalyzers for production of hydrogen using ethanol in fuel cells and for understanding, for example, how oxygen affects the catalyzer,” says Silva.
The next steps for researchers will be to conduct simulations on the molecular dynamics of a platinum nanoparticles, adding oxygen atoms on its surface. They intend to examine whether the structure formed in this process, in which there is an environment of high oxygen concentration, is similar to that observed during Nomiyama’s research.
“His scientific initiation project is integrated with other research projects undertaken by the group. One of our concerns is ensuring that the initiation students are part of the group in order to take the greatest advantage of research activity and guaranteeing that they undertake a well defined project that is both feasible and close to reality,” says Silva.
For Nomiyama, the research project allowed him to broaden his knowledge of the platinum oxides he had already had contact with in some of his graduate courses in chemistry, in addition to working with computational tools and techniques that he would probably only see in post-graduate studies.
“Some characteristics of this chemical element I knew about because of classes I was taking during the research. Others, I only discovered much later. Thanks to basic knowledge of chemistry, it was possible to find these new structures,” he affirmed.
The article Bulk structures of PtO and PtO2 from density functional calculations, by Nomiyama and others (doi:10.1103/PhysRevB.84.100101), was published in Physical Review B, and can be accessed at http://link.aps.org/doi/10.1103/PhysRevB.84.100101.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





