

Immunocytochemical analysis confirms reduction of the expression of the target genes TCF4 and EHMT1 in the silenced lines compared with the control line (RFP) on the scale of 10 mm (images: Elizabeth Chen)
Research indicates that intellectual disabilities and autism spectrum disorders may be caused by alterations of the same molecular pathway.
Research indicates that intellectual disabilities and autism spectrum disorders may be caused by alterations of the same molecular pathway.
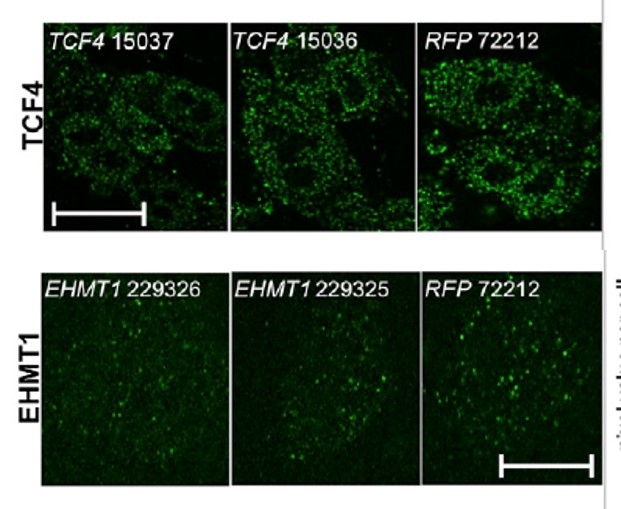
Immunocytochemical analysis confirms reduction of the expression of the target genes TCF4 and EHMT1 in the silenced lines compared with the control line (RFP) on the scale of 10 mm (images: Elizabeth Chen)
By Fabio Reynol
Agência FAPESP – Conditions such as intellectual disabilities and autism spectrum disorders may be caused by alterations of the same molecular pathway. The findings of a related research study were reported in the article “Molecular Convergence of Neurodevelopmental Disorders,” published in October as the cover story of the American Journal of Human Genetics. The findings pave the way for new approaches to how we understand these conditions, known as neurodevelopmental disorders.
“Completely defining the principal molecular pathways by which these syndromes occur offers a viable option for later focusing treatment on this pathway,” said Elizabeth Suchi Chen, a professor in the Department of Morphology and Genetics at the Federal University of São Paulo (Unifesp).
Chen shares principal authorship of the article with postdoctoral fellow Carolina de Oliveira Gigek, of the same institution. FAPESP awarded Chen a scholarship for research abroad to conduct the investigation at McGill University in Montreal, Canada.
These diseases are related to molecular alterations of the neurons that make up the nervous system and human brain during embryonic development. To study these effects, the researchers worked with commercial embryonic neuronal cell lines produced by specialized companies for research purposes.
The team also used cell lines developed in their own laboratory. Fibroblasts, which are skin cells, were reprogrammed to become neuronal precursor cells. This work was carried out by a team led by Prof. Carl Ernst, of the Department of Psychiatry at McGill University, who also wrote the article.
Differentiation into neurons had to be reproduced in the laboratory, and several of the cells studied had reduced expression of two specific genes: TCF4 and EHMT1. “In patients with disorders grouped as a neurodevelopment syndrome, we observed a reduction of the expression of these genes by nearly half; therefore, we modified the cell lines to show a similar reduction,” Gigek said.
After differentiation, the cells with reduced expression were compared with the control group, which had undergone no gene modification, to analyze the molecular modifications that had occurred. The team used next-generation sequencing technology to do this.
“The objective was to determine which molecular alterations would be caused by reducing the expression of these genes in the neurons,” Chen said. The discovery was that when altered separately, these genes caused similar molecular alterations in the cells. This finding led to the conclusion that in isolation, both TCF4 and EHMT1 can generate molecular alterations that lead to similar neurodevelopmental disorders. It was also noted that the same cellular modification could cause different diseases of this type.
According to Chen, what was known before the research study was that many alterations in several genes were associated with neurodevelopmental disorders. “Since then, we observed that reduction of the expression of these two genes led to a convergence of molecular alterations,” she said.
Premature cell differentiation
Another important discovery from the study was the relationship between the reduced expression of the genes studied in the neurons and the fact that they began their process of differentiation prematurely.
“The altered cells appear to present premature differentiation relative to normal cell differentiation, which could be the cause of the problem,” Gigek said. Cell differentiation is the process by which the stem cell acquires the characteristics that will define its function in the body.
The trigger for this premature cell differentiation, however, could not be confirmed in the study. The researchers suspect that a number of RNAs and microRNAs may be involved. “Based on the gene function, this may be suggested. However, further studies will be needed to determine the exact cause,” the postdoctoral candidate said.
Chen believes that the microRNAs associated with cell development are an interesting focus for a future study.
The researchers explained that if the relationship of microRNAs with premature differentiation and the resulting neurodevelopmental disorders is substantiated, it could lead to new options for molecular therapies.
“If the problem is caused by overexpression of RNA, for example, inhibitors can be administered to prevent cells from being stimulated to differentiate early,” Gigek explained, by way of example. She emphasized, however, that this therapy still depends on further research.
The researchers are now planning to validate the results in other neuronal cell lines to confirm their findings. They also need to determine whether the molecular alterations in human development are the same as in the isolated cultures studied in the models. “That is why we need to do a lot more research to confirm the discoveries, but we’ve made considerable progress,” Chen said.
The article “Molecular Convergence of Neurodevelopmental Disorders” (doi: 10.1016/j.ajhg.2014.09.013), by Elizabeth S. Chen, Carolina O. Gigek and colleagues, can be read by subscribers of AJHG at www.cell.com/ajhg/abstract/S0002-9297(14)00396-6.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





