
Therapeutic strategy tested in mice cuts atherosclerosis lesion area by 74% and release of inflammatory molecules by 68% (image: release)
Therapeutic strategy tested in mice cuts atherosclerosis lesion area by 74% and release of inflammatory molecules by 68%.
Therapeutic strategy tested in mice cuts atherosclerosis lesion area by 74% and release of inflammatory molecules by 68%.

Therapeutic strategy tested in mice cuts atherosclerosis lesion area by 74% and release of inflammatory molecules by 68% (image: release)
By Karina Toledo | Agência FAPESP – A nanoformulation capable of removing from the bloodstream the most harmful particles of low-density lipoprotein (LDL), known as “bad cholesterol”, is being tested in mice by researchers at the University of São Paulo (USP) in Brazil.
The study is being conducted at the School of Pharmaceutical Sciences (FCF-USP) as part of a FAPESP Thematic Project, for which Dulcineia Saes Parra Abdalla is principal investigator. Recent results have been published in the European Journal of Pharmaceutics and Biopharmaceutics.
“We propose to create a system to remove electronegative LDL from the bloodstream so as to prevent these particles from interacting with artery walls and inducing a pro-inflammatory response that contributes to the onset and progression of atherosclerosis lesions,” Abdalla told Agência FAPESP.
Although the label “bad cholesterol” is usually applied to the entire LDL fraction, the electronegative subfraction LDL(-), which includes particles modified by chemical processes such as oxidation and lipolysis, can be considered the most atherogenic and hence the main threat to cardiovascular health, according to Abdalla.
When LDL(-) is recognized and internalized by immune cells called macrophages, this sets off an inflammatory response that increases the instability of atheromas (arterial wall plaques) and contributes to their rupture, potentially leading to a cardiovascular event such as acute myocardial infarction (heart attack) or stroke.
“When macrophages internalize these modified lipids, they turn into what we call foam cells,” Abdalla explained. “A buildup of foam cells represents the onset of an atherosclerotic lesion. When these macrophages die, they release all the lipids they contain, along with other cell fragments, forming a necrotic region in the artery wall. More macrophages are attracted to the site, as well as lymphocytes and other kinds of defense cells, and more inflammatory molecules are released, aggravating the situation.”
The group initially set out to create a methodology for detecting the subpopulation of LDL(-) particles in patients with dyslipidemia, diabetes, chronic renal insufficiency and other health problems associated with cardiovascular risk. It is worth recalling that conventional laboratory tests measure LDL as a whole and do not separate out this more atherogenic subfraction.
“We developed monoclonal antibodies capable of identifying and binding only to LDL(-) particles for diagnostic purposes,” Abdalla said. “But then we thought of putting this tool to therapeutic use, in the sense that these antibodies could be deployed to neutralize atherogenic particles.”
Developing the strategy
The studies being conducted by the group at FCF-USP use an experimental model of atherosclerosis with mice that have been genetically modified not to express the gene for the LDL receptor (LDLR). LDL accumulates in the bloodstream of these LDLR-knockout mice, mimicking familial hypercholesterolemia. The buildup of LDL leads to the development of atherosclerosis lesions in these mice, especially in the region of the aortic arch – the portion of the aorta located inside the heart. After less than two months on a cholesterol-rich diet, the mice develop atherosclerosis.
Initially, the group tested treatment with the entire monoclonal antibody, but the immunocomplex that forms when it binds with the antigen LDL(-) could be internalized by macrophages, boosting the formation of foam cells and exacerbating the inflammatory response.
“So, we decided to remove part of the antibody and use only the part capable of recognizing the antigen,” Abdalla said. “In this way, the region of the antibody that binds with the macrophage Fe receptor would be eliminated.”
In partnership with Andrea Queiroz Maranhão, a researcher at the University of Brasília (UnB), the group used genetic engineering to develop a method of expressing in Pichia pastoris yeast a recombinant protein formed by the hypervariable regions of the antibody’s light and heavy chains that recognize LDL(-), which were fused with a linker peptide. Researchers refer to this protein as a single-chain variable fragment (scFv). The anti-LDL(-) scFv was developed by Soraya Megumi Kazuma during her Master’s research, which was supported by a scholarship from FAPESP.
“The problem is that scFv antibodies are small molecules with a short bloodstream half-life compared to the complete monoclonal antibody,” Abdalla explained. “So, the strategy we developed was to include the scFv in a nanocapsule to extend its bloodstream half-life and to enable targeted drug delivery to atheromas in the future.”
Adriana Raffin Pohlmann and Silvia S. Guterres, researchers at the Federal University of Rio Grande do Sul (UFRGS), contributed to this part of the project. Applications for two patents referring to the nanoformulation have been filed with INPI, Brazil’s patent office.
According to Abdalla, the nanoformulation consists of biodegradable multiwall polymeric nanocapsules with a lipid core containing liposoluble drugs coated with multiple layers to which other pharmaceuticals or molecules can be added to deliver the drugs to a therapeutic target.
“Our strategy was to place the scFv on the surface of the nanoparticle so as to deliver it to the LDL(-) present in the bloodstream,” she said. “This would avoid macrophage internalization of the LDL(-) in the nanoformulation. It would also ensure that the scFv antibodies didn’t bind to macrophages via Fe receptors, which would be used by the complete antibody. This would reduce the buildup of cholesterol from LDL modified by macrophages, thereby reducing foam cell formation and the release of pro-inflammatory mediators in the artery wall.”
Proof of concept
In the experimental model, the researchers tested an atherosclerosis-prevention protocol using the nanoformulation. This part of the project was performed during Marcela Frota Cavalcante’s PhD research, which was funded by a scholarship from FAPESP. The mice were injected with a dose seven days before starting on the cholesterol-rich diet (0.5% w/w cholesterol). Four more doses were administered during the 28-day diet period. At the end of the period, histological analysis of the aortic arch region was performed.
In comparison with the control mice, which were given placebo instead of the nanoformulation, the treated mice had a 74% smaller atherosclerosis lesion area and their tissue contained 68% less interleukin-1 beta (IL-1β), a pro-inflammatory cytokine released by macrophages.
“Release of IL-1β, among others, is a major driver of increased inflammatory activity in atherosclerotic plaque, which is more of a problem than the size of the lesion because it increases the risk of atheroma rupture and thrombosis. This is why the main therapeutic strategy today is to combat atherosclerotic plaque inflammation,” Abdalla said.
The nanoformulation acts on two fronts, she added: it removes from the bloodstream the modified LDL that stimulates inflammation, and it minimizes lipid buildup in blood vessels. As a result, it reduces both lesion area and plaque inflammatory activity.
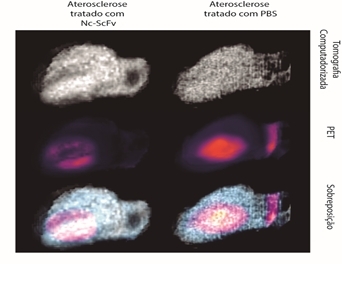
The group’s current studies focus on determining how long the nanoformulation stays in the body before being metabolized and where it delivers the LDL(-) particles. They are using molecular imaging techniques such as positron emission tomography and computed tomography (PET/CT), in collaboration with researchers at the Energy & Nuclear Research Institute (IPEN). The results should be published soon.
The article “A nanoformulation containing a scFv reactive to electronegative LDL inhibits atherosclerosis in LDL receptor knockout mice” (doi: 10.1016/j.ejpb.2016.07.002) can be retrieved from sciencedirect.com/science/article/pii/S0939641116302612.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





