
Brazilian researchers shed light on structural molecular properties of the protein whose functional behavior plays an important role in the development of Autosomal Dominant Polycistic Kidney Disease
Brazilian researchers shed light on structural molecular properties of the protein whose functional behavior plays an important role in the development of Autosomal Dominant Polycistic Kidney Disease
Brazilian researchers shed light on structural molecular properties of the protein whose functional behavior plays an important role in the development of Autosomal Dominant Polycistic Kidney Disease

Brazilian researchers shed light on structural molecular properties of the protein whose functional behavior plays an important role in the development of Autosomal Dominant Polycistic Kidney Disease
Agência FAPESP – Researchers from the Laboratory of Cellular, Genetic and Molecular Nephrology at the University of São Paulo’s Medical School’s Department of Clinical Medicine took an important step toward increasing understanding of the mechanism of an illness that affects one in every 400 or 1,000 people the world over. They shed light on structural molecular properties of the protein whose functional behavior plays an important role in the development of Autosomal Dominant Polycistic Kidney Disease (ADPKD).
The disease, characterized by the formation of multiple cysts (closed liquid-filled sacs) in the kidneys and liver as well as in other organs in many cases, is caused by mutations in the PKD1 (policystic kidney disease 1) gene or the PKD2 gene as result of failed function of the proteins codified by them: respectively, the polycystin-1 (PC1) and plycystin-2 (PC2), whose behavior is controlled by the former.
A non-selective calcium-permeable cation channel, PC2 has a C-terminal intracytosolic tail (PC2t) that plays an important role in its interaction with a number of different proteins, including PC1.
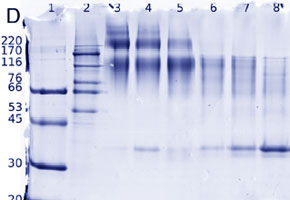
Due to the great importance of this part of the protein, the Brazilian researchers chose to analyze the macromolecular assembly of the protein complex PC2t homo-oligomer using a series of molecular, biophysical and biochemical analyses.
The result of post-doctoral studies by physicist Frederico Moraes Ferreira entitled “Análise estrutural de proteínas relacionadas à doença renal policística autossômica dominante” [ [ Structural analysis of proteins related to autosomal dominant polycistic kidney disease ] , the study was funded by a FAPESP fellowship and published in Proceedings of the National Academy of Sciences magazine.
“It’s a very important study for medicine and biophysics, because it shows structural properties of the C-terminal intracytosolic tail dominium of polycystin-2, which is very important biologically,” said Luiz Fernando Onuchic, coordinator of the Celular, Genetic and Molecular Nephrology Laboratory at FMUSP, one of the authors and coordinators of the research, to Agência FAPESP.
Studies to analyze PC2t were performed at the USP Medical School, the São Carlos USP Physics Institute and at the Brazilian Synchrotron Light Laboratory (LNLS) in Campinas (SP), and included mass spectrometry, x-ray scattering at low angles and dynamic light scattering.
In trials, researchers were able to observe that PC2t is capable of organizing itself as a homotetramer (composed of four protein units) suggesting that this dominium can direct PC2’s molecular assembly. PC2t’s tetrameric arrangement occurs in the presence and the absence of calcium.
The group began a collaborative project with post-doctoral researcher Leandro de Oliveira, at the Universidade de Brasília (UnB) as well as the UC San Diego Theoretical Biological Physics Center in order to perform theoretical analysis and corroborate data. Results of the molecular dynamics simulation, based on experimental data, supported the experimental analyses made by the researchers.
“We demonstrated the molecular organization experimentally and later did molecular dynamics analysis. We obtained results that supported the model we had proposed,” said Onuchic.
According to him, the results of the PC2t molecular model should serve as a jumping off point for beginning to focus on questions about the architecture of PC2 as well as the specific roles it plays in the development of the disease.
“As we begin to shed light on the structural properties of this part of the molecule, its architecture and the functional properties of PC2, we can move forward in understanding the mechanisms of the disease and, consequently, in developing therapeutic intervention,” he affirmed.
As yet, there is still no specific treatment for the disease, but studies are being conducted with some drugs. Estimates say that the illness affects over 600,000 people in the United States and is responsible for 5-10% of all fatal chronic kidney disease where the only options for treatment are dialysis or kidney transplant.
In Brazil, where there are no official data about the number of people affected by the disease, an initial study suggested that it is responsible for 7.5% of fatal chronic kidney disease in the South of the country.
The article Macromolecular assembly of polyscystin-2 intracytosolic C-terminal domain(doi: 10.1073/pnas.1106766108), by Luiz Fernando Onuchic and others can be read by PNAS subscribers at
www.pnas.org
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





