

Compound controls rise in blood pressure in cases associated with sympathetic hyperactivity. Results of preclinical trials are published in Nature Medicine (image: Gray's Anatomy book / Wikimedia Commons)
Compound controls rise in blood pressure in cases associated with sympathetic hyperactivity. Results of preclinical trials are published in Nature Medicine.
Compound controls rise in blood pressure in cases associated with sympathetic hyperactivity. Results of preclinical trials are published in Nature Medicine.
Compound controls rise in blood pressure in cases associated with sympathetic hyperactivity. Results of preclinical trials are published in Nature Medicine (image: Gray's Anatomy book / Wikimedia Commons)
By Karina Toledo | Agência FAPESP – A drug capable of combating arterial hypertension via a novel mechanism has been tested with FAPESP’s support by a group of Brazilian, UK and New Zealand researchers.
The latest results of preclinical trials to test the compound currently known as MK-7264/AF-219 were published in Nature Medicine in September.
Tests in humans are being planned in the UK. If the therapy proves safe and effective, it could also benefit patients with resistant hypertension – blood pressure that remains high despite treatment with currently available antihypertensive medications.
“This new drug acts by blocking P2X3 purinergic receptors present in the carotid body, an organ located in the carotid arteries. These receptors are abnormally activated in individuals with high blood pressure,” said Benedito Honorio Machado, a professor in the Department of Physiology of the University of São Paulo’s Ribeirão Preto Medical School (FMRP-USP) in Brazil.
The drug was developed by Afferent Pharmaceuticals, currently owned by Merck, on the basis of research conducted over the past few decades by groups led by Machado at the University of São Paulo (USP) and Julian Paton, a professor at the University of Bristol in the UK. Their collaborative efforts, lasting over 15 years, include studies to characterize purinergic receptors and cardiovascular control. Scientists at the University of Auckland in New Zealand also collaborated.
According to Machado, the drug prevents activation of P2X3 by adenosine triphosphate (ATP), a molecule that stores energy for cellular activities and one of a number of transmitters involved in hypoxic signaling in the carotid body. This receptor blockade makes carotid body cells, which are hyperactive in people with high blood pressure, return to the pattern of activity considered normal.
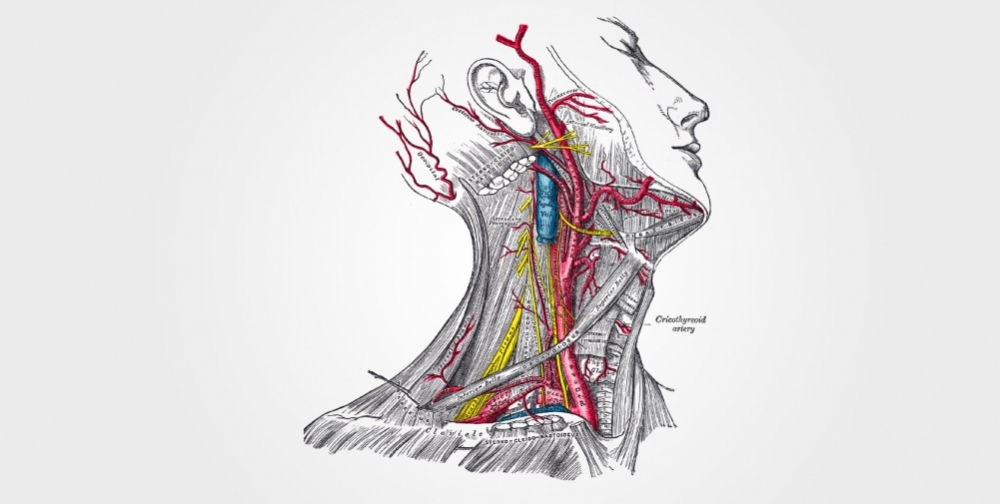
The carotid body is the size of a grain of rice and considered the smallest organ in the human body. In fact, there are two carotid bodies, one in each of the carotid arteries that supply oxygen-rich blood from the heart to the brain, face and neck.
“The carotid bodies are located in the neck region, where the carotid arteries bifurcate,” Machado said. “They act as sensors that warn the central nervous system when oxygen levels in the blood decrease for some reason. When this alarm signal reaches the brain, it triggers a response that includes increased sympathetic activity, which accelerates heart rate and bolsters vascular resistance to blood flow, causing a rise in arterial pressure. It also increases respiration to raise the amount of oxygen reaching the brain.”
This defense system is needed during an episode of obstructive sleep apnea, for example. Oxygen desaturation activates the carotid body cells, which send an alert to the brain.
Under physiological conditions, once blood oxygen levels return to normal, so do sympathetic activity and arterial pressure. However, experiments with rats performed at FMRP-USP have shown that in hypertensive animals these cells constantly send signals to the brain to increase sympathetic activity.
“All the antihypertensive drugs available on the market today interfere with the final effects of this sympathetic hyperactivity, meaning the nerve endings in blood vessels and the heart, inducing vasodilation and a fall in arterial pressure. This new medication will combat hypertension at its source by preventing a rise in sympathetic activity in the first place,” Machado said.
Trials
The experiments conducted by Davi José de Almeida Moraes and Melina Pires da Silva, a professor and researcher, respectively, in FMRP-USP’s Department of Physiology, used spontaneously hypertensive (SH) rats, a strain that becomes hypertensive at about five weeks of age as a result of years of breeding to produce animals with high arterial pressure.
The level of carotid body activity was assessed by electrophysiology, with microelectrodes measuring the level of activity in the cervical ganglion neurons that convey carotid body signals to the brain.
“This assessment of neural activity showed that the hypertensive animals’ carotid body cells were continuously more activated than those of the control animals,” Moraes said. “In addition, when the SH rats were submitted to hypoxia, i.e. oxygen deficiency, their cells responded more strongly.”
To try to discover the cause of this enhanced excitability, the researchers decided to study P2X3 receptors, which they found to be expressed at higher levels in the cells of SH rats and also to over-respond to carotid body application of ATP.
Histological analysis showed that these receptors are also expressed in human carotid body cells.
The next step was to test the effect of P2X3 receptor blockade in animals. The compound MK-7264/AF-219 was first applied directly to the carotid body in SH rats, and both neural and sympathetic activity were found to decrease.
The effect of the medication on arterial pressure was tested by means of acute treatment administered intravenously. The SH rats received a 60-minute infusion of the P2X3 antagonist, and their blood pressure was monitored for an hour thereafter.
“During this period, blood pressure values were similar to those of normotensive animals. Then, they tended to rise back to high levels. To boost the effect in clinical trials, careful planning of the dose for oral administration will be necessary,” Moraes said.
A first sign that the new therapeutic strategy could work in humans is also mentioned by the paper published in Nature Medicine. In an experiment performed in the UK, hypertensive patients received a five-minute intravenous infusion of dopamine, a neurotransmitter that inhibits carotid body activity. The treatment caused a reduction in the volunteers’ respiration, indicating that the carotid bodies are also hyperactive in humans with high blood pressure.
“We believe that in the future this strategy using dopamine could also help identify patients capable of benefiting from P2X3 blockers. Not all cases of hypertension are associated with carotid body cell hyperactivity,” Moraes said.
According to him, surgical removal of one of the carotid bodies has been tested in the UK as treatment for malignant hypertension, extremely high blood pressure that can cause organ damage and cannot be controlled with medication. A reduction in arterial pressure and improvement in quality of life were observed in most patients following the procedure.
“The drawback is the use of surgery in a very delicate region,” Machado said. “In addition, it’s impossible to remove both carotid bodies because the patient would risk death during a sleep apnea episode, for example. Use of the P2X3 antagonist, on the other hand, doesn’t prevent the organism’s alarm system from continuing to function. It merely ceases to be hyperactive.”
Clinical trials are underway with the compound MK-7264/AF-219 for the treatment of chronic cough, which is also associated with purinergic receptor hyperactivity. “The compound has been approved in initial tests to assess toxicity and is now in an advanced stage of clinical trials,” Machado said.
The article “Purinergic receptors in the carotid body as a new drug target for controlling hypertension” (doi: 10.1038/nm.4173) can be retrieved from nature.com/nm/journal/vaop/ncurrent/full/nm.4173.html.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





