

Light microscopy images of S. mansoni after 1 day of treatment with omeprazole and praziquantel (first two columns) and after 7 days of treatment (PLOS Neglected Tropical Diseases)
Researchers have discovered that omeprazole boosts the action of the drug normally used to combat Schistosoma mansoni.
Researchers have discovered that omeprazole boosts the action of the drug normally used to combat Schistosoma mansoni.
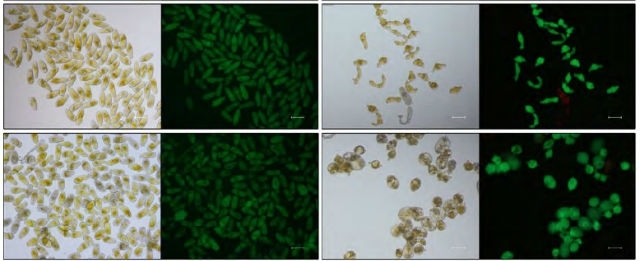
Light microscopy images of S. mansoni after 1 day of treatment with omeprazole and praziquantel (first two columns) and after 7 days of treatment (PLOS Neglected Tropical Diseases)
By Reinaldo José Lopes
Agência FAPESP – Anyone who has recurring stomach trouble is familiar with omeprazole, a drug prescribed by many physicians for gastritis and peptic ulcers, among other digestive disorders. Researchers at the University of São Paulo’s Chemistry Institute (IQ-USP), working with colleagues at the Butantan Institute and other Brazilian institutions, have discovered a novel application of omeprazole using genomic techniques: it boosts the action of the drug normally used to combat Schistosoma mansoni, the worm that causes schistosomiasis.
The discovery, published in the open-access scientific journal PLOS Neglected Tropical Diseases, occurred when the researchers mapped the gene activation pattern in S. mansoni after it had been treated with moderate amounts of praziquantel, a drug whose administration in a single annual oral dose is normally sufficient to eliminate the parasite.
Among the genes that appeared to be associated with the reaction of schistosomes to the drug, there were several DNA regions the equivalents of which in humans are linked to the action of known drugs, and, specifically, one of the most interesting on the list is omeprazole.
According to Sergio Verjovski-Almeida, a professor in the Biochemistry Department at IQ-USP and the principal investigator for the study, the search for drugs capable of acting in synergy with praziquantel derives paradoxically from the success of this medication against schistosomiasis.
Because praziquantel is the only drug used on a wide scale to combat the disease, it is feared that sooner or later lineages of S. mansoni that are resistant to this drug will appear, as is already the case in the arms race between antibiotics and resistant strains of bacteria.
“It’s not yet known for sure whether this resistance has already emerged significantly in the wild. Some possible cases of resistance may be due only to incorrect use of praziquantel. In any event, the concern exists,” Verjovski-Almeida said.
The combined use of two different drugs would greatly reduce this possibility, as the parasite’s organism would have to be “lucky” enough to carry genetic variations capable of making it resistant to both drugs.
Verjovski-Almeida’s search for vulnerabilities in the genes affected by praziquantel was partly inspired by his work in cancer genomics.
“When you use a drug against a tumor, it often happens that the cancerous cells rapidly undergo mutations that can make them resistant to treatment, and cells with these mutations soon produce many clones,” he said. “So why not try to understand these networks of genomic alterations? That’s how I had the crazy idea of working on these two different things at the same time.”
Males, females and protons
To identify the genes affected by the drug, the group first obtained samples of the parasite’s messenger RNA (mRNA). mRNAs are a large family of RNA molecules that convey genetic information from the DNA to the ribosome, where they specify the amino acid sequence of the protein products of gene expression. The more copies of the mRNA corresponding to a given gene that are produced by a cell, the more active the gene will be.
Following this initial step, the mRNA samples were used as a “mold” for the production of their DNA equivalents, which were then added to a microarray with thousands of wells containing probes with copies of S. mansoni’s known gene sections.
The idea behind this process is simple: because DNA molecules are made up of complementary base pairs – base A (adenine) binds only to base T (thymine), and base C (cytosine) binds only to base G (guanine) – the technique ensures that gene sections previously placed on the microarray bind to those active in live parasites.
A visually identifiable marker is added to the wells. The more frequently pairing occurs, the more visible the marker is, so the level of activation of a given gene can be inferred.
The researchers used this technique to try to identify the genes affected by praziquantel in both male and female worms. In the latter case, they analyzed females paired with male mates as well as unpaired females.
The significance of this control is the peculiarity of schistosome mating behavior. When schistosomes of opposite sexes meet, the male holds the slender female in a groove on his underside called the gynecophoric canal, forming a perpetual embrace. “The use of praziquantel can dissolve that embrace. The males typically die first, probably because they’re more directly exposed to the drug as it circulates in the patient’s blood stream,” Verjovski-Almeida said.
All of these factors may influence the dynamics of the disease and its treatment. When considering the two different types of females, for example, praziquantel appears to boost the activation of certain genes in paired female worms but makes almost exactly the same genes less active in unpaired females.
Despite these differences, however, the researchers found that some genes were affected regardless of sex and mating status. One of the genes that most intrigued them was the ATP1A2 gene, which in humans codes for the production of an enzyme that facilitates gastric acid secretion and which is inhibited by omeprazole.
“When I saw that information, I surmised that omeprazole wouldn’t be effective against S. mansoni because the process would occur in the stomach, but in fact, it begins in the blood stream, which is precisely where the parasites are,” Verjovski-Almeida said.
In the case of S. mansoni, the gene probably has nothing to do with gastric acid, but its function is not clear. “What we do know is that it’s associated with the parasite’s reaction to praziquantel,” he explained.
Indeed, in vitro experiments showed that when doses of praziquantel that would not be sufficient to kill the worms were combined with omeprazole, male death rose eightfold and female death threefold.
The next steps are to test the combination of omeprazole and praziquantel in animal models and, if the results again prove encouraging, to try out the same approach in human patients. The advantage is that both drugs are well known and have been used in humans for decades, which theoretically favors their combined use. “We may first perform a toxicity trial in patients,” Verjovski-Almeida said.
In addition to Verjovski-Almeida’s group at IQ-USP, the other participants in the study were researchers at the Butantan Institute in São Paulo; the Oswaldo Cruz Foundation’s René Rachou Research Center in Belo Horizonte, Minas Gerais; and the Vale Technology Institute in Belém, Pará. In fact, Verjovski-Almeida is about to transfer to the Butantan Institute, where he hopes to intensify the applied-science side of research on S. mansoni.
The article “Synergy of Omeprazole and Praziquantel In Vitro Treatment against Schistosoma mansoni Adult Worms” (doi: 10.1371/journal.pntd.0004086), by Sergio Verjovski-Almeida et al., can be read at http://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0004086.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





