

Researchers at the University of São Paulo's Human Genome and Stem Cell Research Center have reconstructed the cellular process that causes Richieri-Costa Pereira syndrome (left: neural crest cells generated from induced pluripotent stem cells from patients with Richieri-Costa-Pereira syndrome / image: Gerson Kobayashi; right: neural crest cells with immunofluorescent staining for vimentin and p75, expressed in this type of cell / image: Luiz Caires)
Researchers at the University of São Paulo's Human Genome and Stem Cell Research Center have reconstructed the cellular process that causes Richieri-Costa Pereira syndrome.
Researchers at the University of São Paulo's Human Genome and Stem Cell Research Center have reconstructed the cellular process that causes Richieri-Costa Pereira syndrome.
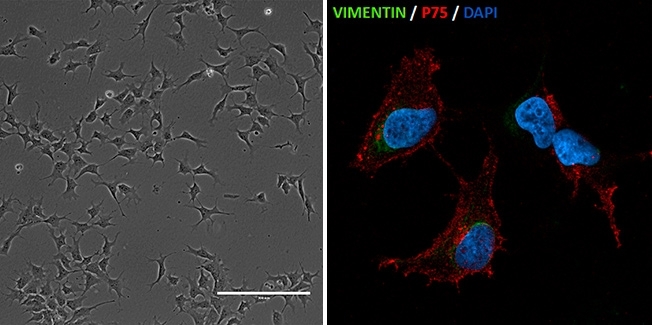
Researchers at the University of São Paulo's Human Genome and Stem Cell Research Center have reconstructed the cellular process that causes Richieri-Costa Pereira syndrome (left: neural crest cells generated from induced pluripotent stem cells from patients with Richieri-Costa-Pereira syndrome / image: Gerson Kobayashi; right: neural crest cells with immunofluorescent staining for vimentin and p75, expressed in this type of cell / image: Luiz Caires)
By Maria Fernanda Ziegler | Agência FAPESP – The cellular and gene expression mechanisms that lead to malformation of the jawbone (or mandible), the most characteristic feature of Richieri-Costa Pereira syndrome, have now been explained.
Researchers at the Human Genome and Stem Cell Research Center (HUG-CELL) in Brazil and at Duke University in the United States have discovered that cases of the syndrome involve problems with the processes of cellular migration and differentiation during the formation of the skull and face in the first trimester of pregnancy.
Richieri-Costa Pereira syndrome is an autosomal recessive condition and was described for the first time in 1992, by Professor Antonio Richieri-Costa and his team, at the Craniofacial Anomaly Rehabilitation Hospital, which is part of the University of São Paulo (USP) and is located in Bauru, São Paulo State, Brazil. Currently, there are more than 20 patients known to have the syndrome in Brazil and one elsewhere, and research on the condition may furnish important clues about craniofacial formation during fetal development and might make a significant contribution to bioengineering.
In 2014, researchers at HUG-CELL, one of FAPESP’s Research, Innovation and Dissemination Centers (RIDCs), which is hosted by USP, discovered that mutations in the gene EIF4A3 are associated with the anomalies characteristic of the syndrome. An article published recently in Human Molecular Genetics describes the cellular mechanisms that lead to the malformation and reconstructs the stages of craniofacial development.
Formation of the head is a complex process comprising several stages. This process requires cell proliferation, cell migration and apoptosis (programmed cell death). The researchers found evidence of a cellular migration problem during craniofacial formation in patients with the syndrome.
“If a migration problem occurs, there will be fewer cells populating the tissues that will be formed, such as the mandible and other parts of the craniofacial complex, for example. We found that a reduction in the number of cells due to defective migration would result in fewer cells to generate these tissues,” said Gerson Kobayashi, one of the authors of the study. Kobayashi’s PhD thesis addressed this topic.
The stages of craniofacial development were reconstructed in vitro using somatic stem cells (dental pulp stem cells, fibroblasts or blood cells) taken from patients with Richieri-Costa Pereira syndrome.
The cells were reprogrammed to become pluripotent stem cells, similar to embryonic stem cells. These were used to create neural crest cells (responsible for generating most cartilage and bone in the craniofacial complex) and mesenchymal stem cells (highly plastic cells that can differentiate into a variety of tissues).
“We used the neural crest cells to observe all three key processes and see whether any of them were compromised in the patients. All was well with proliferation and apoptosis, but not with migration. The important point about neural crest cells is that they should differentiate when they reach a certain region. We tested differentiation into cartilage and bone tissue and confirmed that both processes were compromised,” said Professor Maria Rita Passos-Bueno, who heads the Molecular Disease Diagnosis Laboratory at HUG-CELL.
Better surgery prognosis
The in vitro studies performed at HUG-CELL were replicated in an animal model by researchers at Duke University. Kobayashi believes that the model could be used to search for drugs that reverse the cell phenotype and might be used in high-risk pregnancies. The idea is that the results of the study could serve as a basis for more efficient surgery.
“We’ve seen that the capacity to form bone is there, but it occurs at the wrong time,” Passos-Bueno said. “The next question is whether it would be possible to intervene when surgery is about to be performed because these patients need to undergo several surgical procedures. It might be possible to administer substances that would contribute to more effective bone reconstitution and to a better prognosis for surgery.”
According to Passos-Bueno, discoveries about the mechanisms of Richieri-Costa Pereira syndrome could be relevant to studies in tissue bioengineering.
“The gene EIF4A3 appears to play a key role in formation of the mandible,” she said. “Investment is being made in the production of bone structures in the lab. These could be used to replace compromised bone tissue. If so, we need to identify the critical molecules, for example, to ensure the mandible has a certain size. Long-term application of the knowledge is not only for the patients but also for tissue bioengineering.”
The article “EIF4A3 deficient human iPSCs and mouse models demonstrate neural crest defects that underlie Richieri-Costa-Pereira syndrome,” by Emily E. Miller, Gerson S. Kobayashi, Camila M. Musso, Miranda Allen, Felipe A.A. Ishiy, Luiz Carlos de Caire Jr., Ernesto Goulart, Karina Griesi-Oliveira, Roseli M. Zechi-Ceide, Antonio Richieri-Costa, Debora R. Bertolam, Maria Rita Passos-Bueno and Debra L. Silver, can be retrieved from doi.org/10.1093/hmg/ddx078.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





