
Unesp researchers in Presidente Prudente discover a molecule capable of capturing the atmospheric gas and converting it to compounds that may be used in the future by chemical industries
Unesp researchers in Presidente Prudente discover a molecule capable of capturing the atmospheric gas and converting it to compounds that may be used in the future by chemical industries.
Unesp researchers in Presidente Prudente discover a molecule capable of capturing the atmospheric gas and converting it to compounds that may be used in the future by chemical industries.

Unesp researchers in Presidente Prudente discover a molecule capable of capturing the atmospheric gas and converting it to compounds that may be used in the future by chemical industries
By Elton Alisson
Agência FAPESP – The contribution of excessive emissions of carbon dioxide (CO2) to global climate change has prompted the scientific community to seek more efficient ways to store the compound and reduce its release into the atmosphere.
A new study performed by researchers at the Fine Organic Chemistry Laboratory at the Universidade Estadual Paulista (Unesp) campus in Presidente Prudente opens a window to the development of technology that will allow for the chemical capture of the atmospheric gas and allow it to be converted into useable products for the chemical industry, substituting highly toxic reagents used today in the manufacture of organic compounds used as pesticides or medicines.
Derived from a research project funded by FAPESP through its Young Researchers in Emerging Centers program, the study’s findings, entitled “Study on the fixation and activation of the carbon dioxide molecule with nitrogenized bases” were published in The Royal Society of Chemistry’s Green Chemistry magazine.
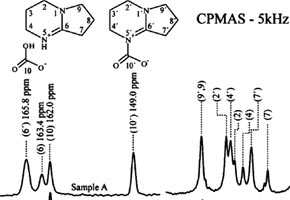
The study showed for the first time that a molecule called DBN (a nitrogenized organic base) is able to capture carbon dioxide, forming compounds (carbamates) that can selectively release CO2 at moderate temperatures. This way, the molecule can be used as a model for research on the selective capture of carbon dioxide from diverse gaseous mixtures.
“This discovery creates new perspectives on how we could make the compound resulting from the bond of DBN with carbon dioxide form in greater quantity. In order to do this, we have to study possible modifications in molecules that show structural and functional similarities to DBN, making the compound more efficient,” said Eduardo René Pérez González, the study’s main author, to Agência FAPESP.
According to the Unesp professor, it was already known that DBN can capture carbon dioxide in the presence of water. In this process, the molecule removes a hydrogen atom from the water molecule, gains a positive charge (proton) and generates hydroxide ions (negative) which attack the carbon dioxide, forming bicarbonates.
Until then, however, it had not been shown that the compound is also capable of capturing CO2, forming carbamate by way of a urethane nitrogen-carbon bond, which is directly related to a biological process in which 10% of the carbon dioxide in the human organism is transported by nitrogenized molecules.
As a result, the process can also be used for the treatment of certain illnesses related to the quantity of CO2 and its transport in the organism.“This discovery makes us think that we could also use this work for biochemical ends. For example, we could try to improve the process for treatment of illnesses related to the concentration of carbon dioxide in the cells of some tissues like lung tissue,” said González.
In industry, carbamates (polyurethanes, for example) derived from the capture of carbon dioxide by the DBN molecule could substitute technology that uses highly toxic reagents like phosgene in the preparation of organic compounds used as pesticides and medicines as well as in other industrial applications.
“Being able to use carbon dioxide to build or synthesize molecules containing the carbonyl group without needing to use phosgene or isocyanates would be a great advantage,” said the research.
The article A comparative solid state 13C NMR and thermal study of CO2 capture by amidines PMDBD and DBN (doi: 10.1039/C1GC15457E), by González and others, can be read by Green Chemistry subscribers at: http://pubs.rsc.org/en/Content/ArticleLanding/2011/GC/c1gc15457e .
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





