
The discovery of the action mechanism of ARHGAP21 in cellular adhesion and migration processes could contribute to the development of techniques that halt the propagation of carcinogenic cells
The discovery of the action mechanism of ARHGAP21 in cellular adhesion and migration processes could contribute to the development of techniques that halt the propagation of carcinogenic cells.
The discovery of the action mechanism of ARHGAP21 in cellular adhesion and migration processes could contribute to the development of techniques that halt the propagation of carcinogenic cells.

The discovery of the action mechanism of ARHGAP21 in cellular adhesion and migration processes could contribute to the development of techniques that halt the propagation of carcinogenic cells
By Elton Alisson
Agência FAPESP – Much like tissues and human organs, tumors are formed by groups of cells that adhere to and interact with other cells. If the adhesion and interaction among tumor cells is weak, there is a greater probability that they will fall apart and migrate to other organs and tissues and begin metastasis (propagation of the cancer).
Researchers from the Hematology and Hemotherapy Center at Universidade Estadual de Campinas (Unicamp) and from the National Institute of Science and Technology on Blood (INCT Sangue), funded by FAPESP, uncovered the role performed by a protein called ARHGAP21 in these cellular adhesion and migration processes.
The results of the study made the cover of the Journal of Biological Chemistry, published by the American Society for Biochemistry and Molecular Biology. These results may contribute to the development of techniques that can “turn off” this protein in tumor cells to impede the emergence of metastasis.
“The biggest problem associated with a tumor is metastasis. If we can block it, it will be possible to impede the propagation of cancerous cells to other organs and increase the chances of a cure,” comments Karin Spat Albino Barcellos, first author of the article.
Barcellos explains that ARHGAP21 was sequenced and described by Professor Sara Teresinha Olalla Saad, coordinator of the National Institute of Science and Technology on Blood, during the Human Genome Project on Cancer, conducted by FAPESP in partnership with the Ludwig Institute and concluded in 2002. At the time, however, the role played by this protein in cells was still unknown.
In the last few years, during the Thematic Project coordinated by Saad, Barcellos and other participating researchers discovered that ARHGAP21 regulates the cytoskeleton (responsible for maintaining the shape of cells and cellular junctions) and acts on Rho-GTPase proteins – a group of approximately 20 proteins that regulate movement, adhesion, migration and cellular differentiation.
“We found that the Rho-GTPases need ARHGAP21 during the formation of cell-to-cell adhesion and that ARHGAP21 participates in this process by staying between the cellular junctions. Some hours after this process is concluded, it leaves. This is why we still haven’t managed to see the presence of ARHGAP21 in the cell-to-cell adhesion process,” explained Barcellos.
To observe the behavior of ARHGAP21 in the cellular adhesion and migration processes, the group conducted laboratory experiments that simulate the development of metastasis.
Known as the epithelial-mesencymal transition, the in vitro metastasis simulation technique has already been explored by research groups in other countries, such as the Department of Physiology and Biological Development at Brigham Young University in Utah.
Barcellos became familiar with the technique while performing an internship in Utah with technical reserves from her post-doctoral project under a FAPESP fellowship and under the auspices of the Thematic Project coordinated by Saad. She then decided to replicate the technique to analyze the functions of ARHGAP21 in cellular adhesion and migration upon returning to Brazil.
One of the hypothesis before beginning the experiment was that, because ARHGAP21 plays a strategic role in cellular adhesion, by removing it from human prostate cancer cells during lab tests its migration, and consequently the metastasis, would be much greater than that observed in carcinogenic cells with the protein.
Upon injecting the cancerous cells (without ARHGAP21) with fractions of HGF, a hormone produced mainly by the liver that makes the cells separate from one another to form organs and tissues in the embryonic phase, the researchers found that the cells did not move, and metastasis did not occur.
“In the beginning, we thought we were making a mistake in some phase of the experiment, like forgetting to inject HGF. However, we repeated the experiment several times and we saw that, in fact, without the ARHGAP21, the cancer cells do not break away and do not migrate. This result surprised us,” affirmed Barcellos.
The researchers noted that in reality ARHGAP21 is located in the HGF signaling pathways of cells and regulates epithelial-mesenchymal transition. The cells without the protein in the HGF signaling pathway, for example, sense the presence of the hormone but cannot break away from the others.
“We showed that it is possible to block induced metastasis induced by HGF through the inactivation of ARHGAP21 through in vitro tests,” affirmed Barcellos. “We still don’t know, however, if it is possible to deactivate this protein in humans because it must exercise many other functions, including beneficial functions in cells.”
Through the new Thematic Project, also supported by FAPESP and coordinated by Professor Sara Saad, the researchers intend to conduct simulations of several types of tumors in mice with cancer cells without ARHGAP21.
“It will be very important to test this in mice and see if it works in order to evaluate the possibility of utilizing the technique in humans to block metastasis,” said Barcellos.
“It may be necessary to remove ARHGAP21 from only cells with tumors or to block the site of the protein that feels the HGF so that the protein can perform other beneficial functions,” she suggests.
LaCTAD’s contributions
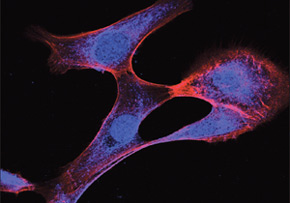
According to Barcellos, some of the main images of the experiments – which illustrate the cover of the January edition of the Journal of Biological Chemistry – were captured using a new confocal microscope, acquired by Unicamp with the support of FAPESP’s Multiuser Equipment Program.
The microscope, in addition to other equipment the university acquired through the project, is housed at the Central Laboratory of Technology in Life Sciences (LaCTAD), inaugurated at Unicamp this past March.
The multiuser unit was conceived along the lines of existing facilities at research centers abroad. The laboratory has cutting-edge equipment for researchers to conduct experiments in areas of genomics, bioinformatics, proteomics and cellular biology.
The laboratory’s instrumental park had investments of approximately R$ 6 million. Unicamp contributed construction of the building and hiring of the personnel. “Unicamp’s scientific community was anxious to have a multiuser laboratory with a centralized heavy infrastructure and institutional support to have personnel who are trained to operate the equipment,” said Professor Sara Saad, a member of LaCTAD’s administrative board.
“It would not be possible to make the image used to illustrate the cover of The Journal of Biological Chemistry, where the epithelial-mesenchymal transition is visible, if not for the expertise of the person who operated the equipment and analyzed the images, who was contracted by LaCTAD, in addition to the researchers and the microscope acquired with FAPESP resources,” states Sara Saad, who is also one of the authors of the article.
For Barcellos, the possibility of using equipment like the confocal microscope LaCTAD used to analyze the role of ARHGAP21 of cellular adhesion was fundamental both for the discoveries they made in the study and to certify that the results were correct.
“If we had not used the latest generation confocal microscope like the one utilized to obtain the images, we could have had doubts, for example, about whether the protein is located in the cytoplasm or the cell nucleus,” explained Barcellos.
“By having equipment like this, we could be certain that ARHGAP 21 migrates from the nucleus of the cancer cell during metastasis, as the image used on the cover of The Journal of Biological Chemistry illustrates,” she affirmed.
The article “ARHGAP21 protein, a new partner of α-tubulina involved in cell-cell adhesion formation and essential for epithelial-mesenchymal transition” (doi:10.1074/jbc.M112.432716), by Barcellos and others, can be read by subscribers of the Journal of Biological Chemistry at www.jbc.org/content/288/4/2179.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





