

Cells genetically modified to express a light-emitting enzyme serve as tools to monitor tumor repopulation (photo: release)
Studies suggest that combining radiotherapy with drugs that block a protein called PAF-R may increase the treatment success rate and reduce relapses.
Studies suggest that combining radiotherapy with drugs that block a protein called PAF-R may increase the treatment success rate and reduce relapses.
Cells genetically modified to express a light-emitting enzyme serve as tools to monitor tumor repopulation (photo: release)
By Karina Toledo | Agência FAPESP – Whenever an organism is damaged, be it by a simple cut on a finger or surgery, the cells surrounding the wound receive signals to proliferate more intensely so as to regenerate the injured tissue.
In the case of cancer, the process is no different. Tumor cells may be all but eliminated by radiation therapy and/or chemotherapy, only to return even more aggressively some time later.
In a project supported by FAPESP with Professor Sonia Jancar as principal investigator, researchers at the University of São Paulo’s Biomedical Science Institute (ICB-USP) in Brazil showed that a protein called PAF-R (platelet activating factor receptor) plays a key role in the phenomenon of tumor repopulation, at least for the types of cancer studied by their group.
Some of their results were recently published in Oncogenesis.
“In experiments with tumor cell lines and mice, we found that PAF-R-blocking drugs significantly inhibited tumor growth and repopulation after radiotherapy,” Jancar told Agência FAPESP. “We therefore suggested associating radiotherapy with antagonists of this receptor as a promising new therapeutic strategy.”
According to Jancar, this line of research began in the 1990s during Denise Fecchio’s PhD studies, when the group showed that tumors induced in the peritoneal cavity of mice grew significantly less when PAF-R was blocked. In that project, the researchers found that treatment with PAF-R antagonists activated macrophages (a type of defense cell) and inhibited tumor growth. The results were published in Inflammation.
Some years later, during Soraya Imon de Oliveira’s PhD research in collaboration with Roger Chammas, a professor in the University of São Paulo’s Medical School (FM-USP), the group showed that tumors also grew less in mice treated with PAF-R antagonists. The results were published in BMC Cancer.
More recently, during Ildefonso Alves da Silva-Junior’s PhD research, the group proved that activation of PAF-R induced proliferation of tumor cells and protected them against death in response to radiation therapy. The research was supervised by Ana Paula Lepique, a professor at ICB-USP, and Chammas’s team collaborated.
“Silva-Junior showed that radiation led to the production of molecules similar to PAF, which activated PAF-R in tumor cells, driving increased expression of PAF-R and tumor cell proliferation,” Jancar said. “So this activation promoted tumor repopulation. Using more sensitive methods, the study also confirmed that when the macrophages present in the tumor microenvironment were treated with PAF-R-blocking drugs, they were reprogrammed to combat the disease more effectively.”
Preclinical trials
The experiments that proved the involvement of PAF-R in tumor repopulation were performed with human oral cancer cells and murine cervical cancer cells. Radiotherapy is usually preferred for treating these kinds of cancer.
The tumor cells were placed in culture medium and irradiated after they began to grow, to simulate radiotherapy. Large numbers of molecules similar to PAF-R were produced in the cultures shortly afterward.
“PAF is actually a phospholipid produced mainly in inflammatory and cell death processes,” Silva-Junior explained. “Radiotherapy kills tumor cells by inflammation, so levels of PAF increase strongly as a result.”
The researchers then treated some of the cultured cells with PAF-R-blocking drugs. Various molecules were tested, including some that were already commercially available but had never been used against cancer.
Analysis performed shortly after treatment showed up one-third more death from radiotherapy among the cells exposed to PAF-R antagonists than among untreated cells. Another analysis performed nine days later showed a much higher rate of cell proliferation in untreated lines, which multiplied about 1.5 times as much as cells treated with PAF-R antagonists.
Next they injected irradiated tumor cells under the skin of mice to observe the phenomenon of tumor repopulation in vivo, i.e., in a situation where there was interaction with immune cells and other factors. At about 30 days after the cells were injected, the researchers measured tumor volume.
“In this experiment, we used two lines of genetically modified tumor cells,” Silva-Junior said. “One overexpressed PAF-R. The other didn’t express PAF-R at all. Tumor repopulation only occurred in the mice that received the line with PAF-R overexpression.”
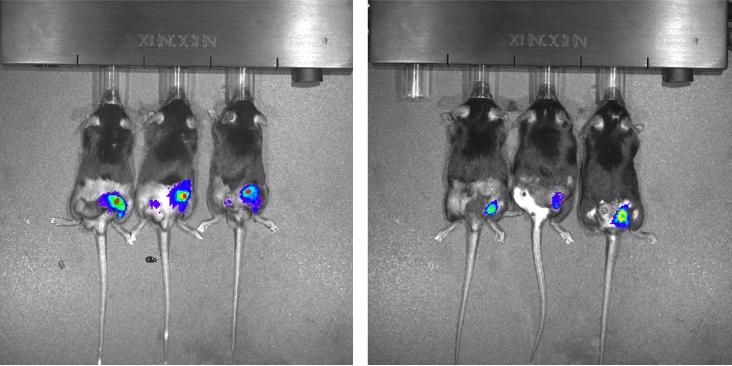
In a third experiment, mice were injected with tumor cells and a type of control cell genetically modified to express a light-emitting enzyme that served as a marker inside tumors.
“The control cells were not irradiated, but they were exposed to the same environment and received the signals produced by the tumor to stimulate cell proliferation,” Silva-Junior said. “We used an in vivo imaging system, or IVIS, to measure the proliferation of these luminescent cells and calculate the extent to which irradiation was inducing tumor repopulation.”
The results showed that the proliferation rate in the animals injected with irradiated cells that overexpressed PAF-R was 30 times higher than in those injected with non-irradiated cells of the same line.
Next steps
To find out whether the results were specific to the tumor cell lines studied to date, the researchers at ICB-USP are now replicating the experiments with ten other kinds of human tumor and performing trials in which PAF-R antagonists are tested in combination with chemotherapy drugs.
The researchers are also testing new kinds of PAF-R inhibitors on the tumor cell lines, including a group of molecules isolated from a marine fungus by Professor Roberto Berlinck and his team at the São Carlos Chemistry Institute (IQSC-USP) in the interior of São Paulo State.
“Several of these molecules have proved to be powerful PAF-R antagonists and capable of inhibiting tumor repopulation,” Jancar said. “Although the discovery is important, the road to validation and use in clinical trials is long and requires collaboration among researchers in basic science like us, chemists to synthesize molecules, and clinicians to test them in healthy subjects and in patients.”
The ideal approach would be to return to clinical trials of PAF-R antagonists performed in the 1980s with patients with asthma or pancreatitis, Jancar added. “For these diseases, the trials were negative, but they could be positive against cancer. I hope our publications alert other researchers in the same field so that they can take this step. Meanwhile, we’re evaluating ways of protecting our findings,” she said.
The article “Platelet-activating factor (PAF) receptor as a promising target for cancer cell repopulation after radiotherapy” can be read at nature.com/oncsis/journal/v6/n1/full/oncsis201690a.html#aff1.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





