
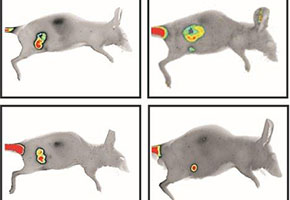
Images compare tumor size in the control group (top line) and in the group that received INXS on the first and 15th day of treatment. The experiment was published in the journal Nucleic Acids Research (images: S. Verjovski-Almeida)
Discovery published in the journal Nucleic Acids Research paves the way for the development of new cancer treatments.
Discovery published in the journal Nucleic Acids Research paves the way for the development of new cancer treatments.

Images compare tumor size in the control group (top line) and in the group that received INXS on the first and 15th day of treatment. The experiment was published in the journal Nucleic Acids Research (images: S. Verjovski-Almeida)
By Karina Toledo
Agência FAPESP – Researchers from the University of São Paulo (USP) have identified an RNA known as INXS that, although containing no instructions for the production of a protein, modulates the action of an important gene in the process of apoptosis, or programmed cell death.
According to Sergio Verjovski-Almeida, professor at the USP Chemistry Institute and coordinator of a research funded by FAPESP, INXS expression is generally diminished in cancer cells, and methods that are capable of stimulating the production of this non-coding RNA can be used to treat tumors.
In experiments on mice, the USP scientists were able to effect a 10-fold reduction in the volume of subcutaneous malignant tumors by administering local injections of a plasmid – a circular DNA molecule – containing INXS. The findings were published in the most recent issue of the journal Nucleic Acids Research.
The group headed by Verjovski-Almeida at USP has devoted the past five years to investigating the regulatory role of so-called intronic non-protein-coding genes – those found in the same region of the genome as a coding gene but on the opposite DNA strand. INXS, for example, is an RNA expressed on the opposite strand of a gene coding for a protein known as BCL-X.
“We were studying several protein-coding genes involved in cell death in search of evidence that one of them was regulated by intronic non-coding RNA. That was when we found the gene for BCL-X, which is located on chromosome 20,” he explained.
The researcher explained that BCL-X is present in cells in two different forms: one that inhibits apoptosis (BCL-XL) and one that induces the process of cell death (BCL-XS). The two isoforms act on the mitochondria but in opposite ways. The BCL-XS isoform is considered a tumor suppressor because it activates protein complexes known as caspases, which are required for the activation of other genes that cause cell death.
“In a healthy cell, there is a balance between the two BCL-X isoforms. Normally, there is already a smaller number of the pro-apoptotic form (BCL-XS). However, in comparing tumor cells to non-tumor cells, we observed that tumor cells contain even fewer of the pro-apoptotic form, as well as reduced levels of INXS. We suspect that one thing affects the other,” the researcher said.
To confirm the hypothesis, the group silenced INXS expression in a normal cell lineage and the result, as expected, was an increase in the BCL-XL (anti-apoptotic) isoform. “The rate between the two – which was 0.25 – decreased to 0.15; in other words, the pro-apoptotic form that previously represented one fourth of the total began to represent only one sixth,” Verjovski-Almeida explained.
The opposite occurred when the researchers artificially increased the amount of INXS using plasmid expression in a kidney cancer cell line, with the non-coding RNA being reduced. “The pro-apoptotic form increased, and the anti-apoptotic form decreased,” the researcher noted.
The next step was to subject the cancer cell lineages to agents known to induce cell death, such as ultraviolet light and chemotherapy drugs, to see whether INXS expression increased.
“We used three agents that induced cell death via mitochondria and determined that all of them caused an increase in the proportion of the pro-apoptotic isoform and a reduction in the anti-apoptotic isoform. We also noted a 5 to 10-fold increase in the level of INXS expression and an increase in the activity of caspases – markers of the apoptosis process,” Verjovski-Almeida explained.
The researchers repeated the experiment, but his time silenced the INXS gene. They then observed that, even in the presence of ultraviolet light, the pro-apoptotic isoform did not increase and the cells did not die. “This provided more evidence that INXS is in fact the mediator of this process,” Verjovski-Almeida affirmed.
The final stage of the study was to verify whether the increase in INXS expression is related to the death of cancer cells in vivo. To do this, the researchers subcutaneously implanted human kidney cancer cells in mice and waited 40 and 60 days for the tumor to reach a volume of 300 cubic millimeters (mm3) and become palpable.
The animals were then divided into two groups: one half began to receive injections of the plasmid containing INXS at the site of the tumor; the other half, which served as the control, received only the empty plasmid.
After 15 days of treatment, the tumors in the control group animals had grown to an average volume of 600 mm3. However, in the group treated with INXS, the average tumor volume measured 70 mm3 – nearly 10 times smaller.
“The tumors in the animals that received INXS were not only smaller and lighter but also whitish, which is a sign of reduced vascularization at the site. In addition, when we measured the ratio between the BCL-X isoforms, the treated tumors had a larger proportion of the pro-apoptotic isoform, suggesting that the remaining tumor cells were already beginning to die,” the researcher explained.
According to the assessment of Verjovski-Almeida, it is possible to develop therapies to fight cancer that are able to increase the quantity of INXS in only the tumor cells, and the USP group plans to test some of these strategies in the future.
In addition to this, in a new thematic project, titled “Characterization of the mechanisms of action of long non-coding RNA involved in the programs of gene activation in human cells,” recently approved by FAPESP, the group plans to further study the mechanisms through which INXS modulates the BCL-X gene to understand why this non-coding RNA is reduced in cancer cells.
The experiments published in the journal Nucleic Acids Research were carried out during the doctoral studies of Carlos De Ocesano-Pereira, a FAPESP scholarship recipient. They also included the participation of post-doctoral fellows Murilo Sena Amaral and Kleber Simônio Parreira.
The article “Long non-coding RNA INXS is a critical mediator of BCL-XS induced apoptosis” (doi: 10.1093/nar/gku561) may be read at: nar.oxfordjournals.org/content/early/2014/07/03/nar.gku561.abstract.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





