

Researchers from the University of São Paulo and the University of California, Berkeley, found that Xylella fastidiosa produces nanoscale lipid particles to travel and spread more easily in plants (image: Paulo Zaini)
Researchers from the University of São Paulo and the University of California, Berkeley, found that Xylella fastidiosa produces nanoscale lipid particles to travel and spread more easily in plants.
Researchers from the University of São Paulo and the University of California, Berkeley, found that Xylella fastidiosa produces nanoscale lipid particles to travel and spread more easily in plants.
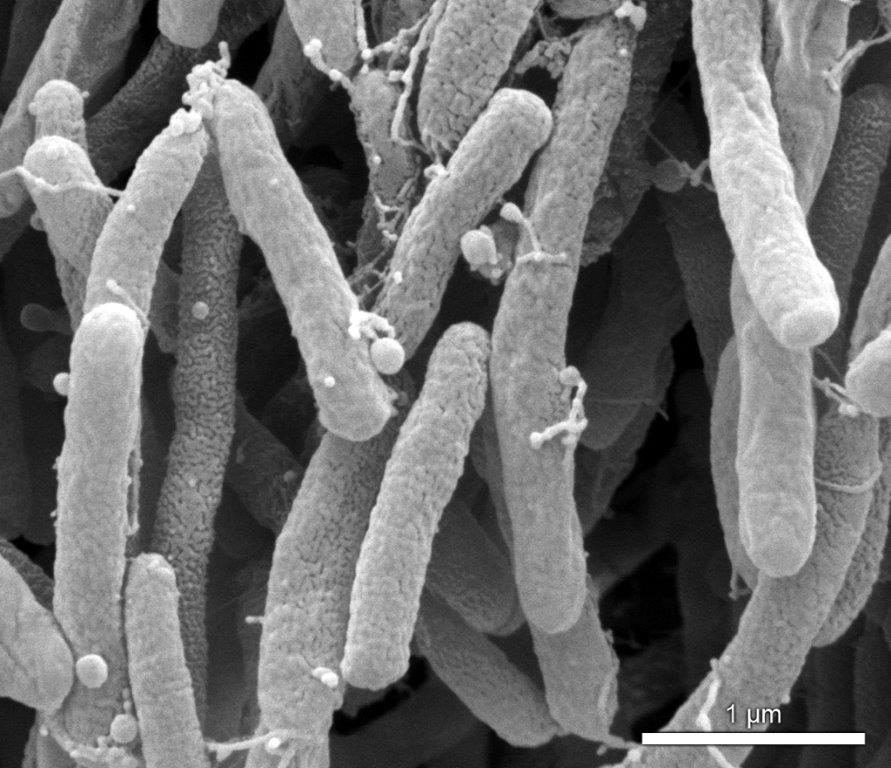
Researchers from the University of São Paulo and the University of California, Berkeley, found that Xylella fastidiosa produces nanoscale lipid particles to travel and spread more easily in plants (image: Paulo Zaini)
By Elton Alisson
Agência FAPESP – The bacterium Xylella fastidiosa, which causes a number of diseases in citrus, vine and coffee plants, has adopted several adaptation strategies to survive in the environment in which it lives.
In order to colonize the buccal apparatus of insect vectors and contaminate plants while these insects feed on the sap of agricultural crops, the bacterium manifests a “sticky” or adhesive state. Once a plant is inoculated by the insect vector, the bacterium then assumes a sticky state to travel and spread more easily inside the xylem vessels (tissue) of the plant.
“Xylella fastidiosa leads a double life. It has to alternate between these two adaptation strategies in order to survive,” said Aline Maria da Silva, professor at the Chemistry Institute (IQ) of the University of São Paulo (USP), in comments to Agência FAPESP.
A study carried out by researchers led by Silva in the Biochemistry Department of the IQ, together with colleagues from the Department of Plants and Microbial Biology of the University of California (UC), Berkeley, in the United States, has demonstrated that Xylella fastidiosa regulates the transition of these adaptation strategies by producing lipid vesicles (bubbles) with dimensions on the nanoscale (one billionth of a meter).
The outcome of a FAPESP-funded postdoctoral research project under the advisorship of Silva, the discovery was described in an article published in the journal Proceedings of the National Academy of Sciences (PNAS).
The article was recommended by the Faculty of 1000, a U.S. website that identifies and recommends articles deemed significant for biology and medicine based on suggestions made by a committee of scientists and physicians from various countries.
“In the article, we proposed that production of the outer membrane vesicles by Xylella fastidiosa is a type of fine-tuning that the bacteria engages in to adhere, or not, to the environment it colonizes, depending on the circumstances,” Silva said.
Recently, the UC Berkeley group of researchers observed that Xylella secreted a type of substance that blocked the bacterium’s ability to adhere to the walls of plant xylem. However, they did not know exactly what the substance was.
Through collaborative efforts begun in 2011 with UC Berkeley professor Steven Lindow, the researchers worked together to investigate the nature of the substance that gives the bacteria this “Teflon” effect.
The prime suspects were the outer member vesicles because Gram-negative bacteria such as Xylella fastidiosa are constantly secreting small lipid bubbles that range from 20 to 100 nanometers in size, Silva explained.
“The function of these outer membrane vesicles in other bacteria has already been studied extensively,” she said. “In the case of the human pathogens, they perform the job of carrying proteins, virulence factors and antigens during infections. However, in phytopathogens [disease-causing organisms in plants], such as Xylella fastidiosa, studies are just beginning.”
In an effort to fill this gap, the researchers used a variety of analytical techniques to assess whether Xylella fastidiosa would also produce outer membrane vesicles, what the size of the bubbles would be in relation to the bacteria and, in particular, what role these bubbles would play.
The results of the analyses indicated that the outer membrane vesicles do in fact provide the bacteria with nonstick properties.
When in the buccal apparatus of insect vectors, Xylella fastidiosa produces a number of outer membrane vesicles that are just large enough to allow the bacterium to break loose from the animal and infect plants through the sap on which the animal feeds.
Once it has infected the plant, however, the bacterium begins to produce larger numbers of these vesicles in order to invade and move about the xylem vessels.
After colonizing the plant to a sufficient extent, the bacteria stop producing the outer membrane vesicles so that they are then able to connect to other bacteria, forming a biofilm inside the xylem vessels of the plant that increases the bacteria’s virulence.
“The bacterium needs to find a way to spread out through the plant after it infects it. And the outer membrane vesicles are one weapon it uses to attack the plant,” Silva noted.
Mechanism of action
The researchers determined that one of the mechanisms used by Xylella fastidiosa to control the production of outer membrane vesicles and the transition from the “sticky” phase to the Teflon phase is a molecule known as DSF (diffusible signal factor).
DSF signals to Xylella fastidiosa how many bacteria similar to it are in the environment. When moving into a microenvironment such as the intestines of an insect vector or the xylem of a plant, the bacterium produces and releases DSF. Based on the amount of DSF already in the environment—produced by other bacteria that are similar to it—Xylella fastidiosa is able to estimate the density of its population.
If there are already numerous bacteria isolated in the environment, the bacteria collectively diminish the production of outer membrane vesicles and produce adhesion molecules to form a biofilm that allows them to survive the adverse conditions found in the environment.
Similarly, the bacteria are able to perceive, through the release of DSF, whether their numbers in the environment are low, in which case they increase the production of outer membrane vesicles to further explore a given site, Silva explained.
“We have found that the more bacteria there are in a microenvironment, and the more together they are, the lower the production of outer membrane vesicles,” the researcher said. “On the other hand, the more isolated the bacteria are, the higher the production of outer membrane vesicles, to allow them to spread more easily.”
This conclusion was drawn by using a strain of Xylella fastidiosa that lacks one gene that is essential to the production of DSF–the mutant ΔrpfF–developed by the group of researchers from UC Berkeley through genetic engineering techniques.
The researchers observed that this mutant strain of the bacteria produced five times as many membrane vesicles as that produced by the non-genetically modified bacteria.
“The more outer membrane vesicles the mutant bacteria produced, the more virulent they were. They stuck together less and spread more quickly and efficiently through the plant, which thus became increasingly more contaminated,” Silva said.
Innovative analytical techniques
The number of outer membrane vesicles produced by Xylella fastidiosa was estimated by analyzing the sap from plants contaminated by wild and mutant bacteria.
To identify and quantify vesicle production, the researchers used the protein marker XadA1, which reveals the presence of vesicles in a solution, such as plant sap.
Using scanning electron microscopy (a technique capable of producing high-resolution images of the surface of a sample) and fluorescence microscopy, the researchers were immediately able to observe the outer membrane vesicles interacting with surfaces and with the bacteria in plant sap.
In addition to more conventional analytical techniques, the researchers also used methodologies that are new in studies of this nature, such as nanoparticle tracking analysis (NTA), said Paulo Adriano Zaini, postdoctoral fellow under the advisorship of Silva, who shares first authorship of the article with UC Berkeley postdoctoral fellow Michael Ionescu.
“We are one of the first groups of researchers to use this analytical method that allows us to track nanoparticles in a solution,” he said.
The methodology was then used to study vesicles in human cells, such as tumors, which produce vesicles in large quantities to allow them to move more easily, Zaini explained. In the study, the researchers used equipment from the A.C. Camargo Cancer Center.
“This is a very original approach for studying bacteria. We think that it will have an influence on other bacteria research groups,” he noted.
The researchers also used a technique called microfluidics, in which Zaini specializes. The technique allows for plants’ xylem vessels to be simulated using microscopic glass cannulas measuring 50 micrometers in diameter.
By using this technique, the researchers measured the force with which the bacteria are uprooted from the surface of the cannulas in the absence or presence of outer membrane vesicles.
“We were able to demonstrate that, in the absence of outer membrane vesicles on the surface of the cannulas, the bacteria were able to withstand a very high speed of sap flow and remain attached to the glass,” Zaini said. “However, on the cannulas covered with membrane vesicles, the bacteria came away much more quickly.”
The article, Xylella fastidiosa outer membrane vesicles modulate plant colonization by blocking attachment to surfaces (doi: 10.1073/pnas.1414944111), by Zaini and colleagues may be read in the journal PNAS at: www.pnas.org/content/111/37/E3910.abstract.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





