
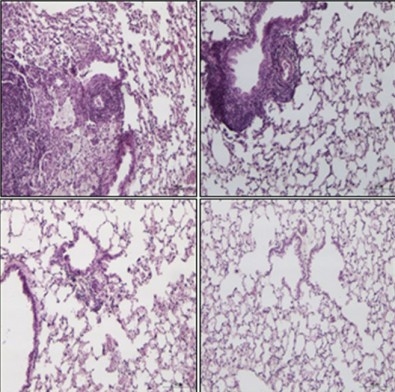
A TB vaccine being developed in Brazil has been successfully tested in mice. Experiments are described in Scientific Reports (image: lung tissue from non-immunized mice (top left), mice vaccinated with conventional BCG (top right), and mice given recombinant vaccine (bottom left and right))
A vaccine being developed in Brazil has been successfully tested in mice. Experiments are described in Scientific Reports.
A vaccine being developed in Brazil has been successfully tested in mice. Experiments are described in Scientific Reports.

A TB vaccine being developed in Brazil has been successfully tested in mice. Experiments are described in Scientific Reports (image: lung tissue from non-immunized mice (top left), mice vaccinated with conventional BCG (top right), and mice given recombinant vaccine (bottom left and right))
By Karina Toledo | Agência FAPESP – A new vaccine against tuberculosis, which is more powerful than the BCG vaccine currently used to immunize children, is being developed at the Butantan Institute (São Paulo, Brazil) with FAPESP’s support.
Promising results of preclinical trials in mice have been published in Scientific Reports, an online journal owned by Springer Nature.
“Traditional BCG vaccines are effective to protect children from the most severe forms of the disease but afford limited protection against adult pulmonary infection. The development of a more powerful vaccine has challenged the international scientific community. Several strategies are being tested,” said Luciana Leite, the head of Butantan Institute’s Special Vaccine Laboratory and the principal investigator for the project.
The strategy used by the São Paulo group focuses on developing a recombinant version of BCG. This entails modifying Mycobacterium bovis, the bacterium used to formulate the conventional vaccine, so that it produces a protein typical of another bacterium, Escherichia coli.
“This recombinant protein, which we call LTAK63, acts as an adjuvant in the formulation, meaning it boosts the immune system’s response to the vaccine,” Leite explained.
In experiments with mice, the researchers compared the protection provided by conventional BCG with that provided by recombinant BCG. The control group comprised non-immunized mice.
Twelve weeks after vaccination, all three groups were infected with Mycobacterium tuberculosis, the bacterium that causes tuberculosis. After 30 days, the number of bacteria present in the lungs was estimated.
In the non-immunized mice, histological analysis showed massive infiltration of inflammatory cells into the lungs, and the number of bacteria in the pulmonary tissue reached 1 million. In the group given conventional BCG, the bacterial load was approximately 100,000, and the degree of inflammation was more moderate, albeit greater than in the group given the recombinant version of the vaccine. Only about 1,000 bacteria were found in the lungs of this third group.
“Next, we performed a second experiment in which we challenged the mice with up to 100 times the original amount of M. tuberculosis and found that only the recombinant version of BCG afforded protection in this case. The group that received the conventional vaccine began dying after only a few days,” Leite said.
Shortening phases
According to Leite, the development of this new vaccine against tuberculosis follows a previous research project that was aimed at creating a recombinant version of the DTP vaccine against diphtheria, tetanus and pertussis.
The project began in 2000, when, with FAPESP’s support, Leite set up a laboratory to develop the methodology required for the production of recombinant BCG.
“Because it induces a strong non-specific immune response in the organism, the bacterium used in BCG vaccine has also been used as an adjuvant in cancer treatment and immunization against several diseases,” Leite said. “Our idea at that time was to develop a recombinant version of this microorganism that could produce, for example, a protein from the bacteria that cause pertussis. So that it would be possible to immunize people against both diseases.”
This initial project is at an advanced stage. According to Leite, Phase I clinical trials of the recombinant vaccine against pertussis will begin soon, with financing from BNDES, the national development bank.
During preclinical experiments, the group found that the recombinant protein used for immunization against pertussis also exerted an adjuvant effect on BCG, modifying the immune system’s response to tuberculosis.
“We observed that the response was different, but not sufficient to increase protection against tuberculosis, which is caused by a highly virulent bacterium,” she said. “So we began looking for a different protein that could boost the immune response against tuberculosis even more strongly. That’s how we came across LTAK63.”
The good news, she added, is that much of the knowledge acquired during the pertussis project can be used in developing the new vaccine against tuberculosis, thereby shortening crucial phases.
“We took many years to adapt the formulation tested in mice for use in humans in the case of the pertussis vaccine. But now we have the methodology ready, and the process will be faster,” she said.
The work began during the postdoctoral research of Ivan Pereira Nascimento with FAPESP’s support and is continuing during the PhD research of Carina Carvalho dos Santos, who is currently engaged in a research internship at Leiden University Medical Center in the Netherlands, also with FAPESP’s support. The research has already won patents in the United States and South Africa.
The new tuberculosis vaccine should be ready within 10 years. According to the World Health Organization (WHO), tuberculosis affects more than 10 million people worldwide, and Brazil is one of the 30 countries with the highest incidence of the disease.
The article “Recombinant BCG expressing LTAK63 adjuvant induces superior protection against Mycobacterium tuberculosis” can be read at: www.nature.com/articles/s41598-017-02003-9.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





