

Sections of brown adipose tissue from mice kept at thermoneutrality or cold for 14 days, revealing tissue remodeling at different temperatures (image: Osvaldo Rodrigues Pereira Júnior et al./American Journal of Physiology-Cell Physiology)
Research conducted by Redoxoma, a FAPESP Research, Innovation, and Dissemination Center, found that mitochondrial potassium channels regulate heat production in brown adipose tissue.
Research conducted by Redoxoma, a FAPESP Research, Innovation, and Dissemination Center, found that mitochondrial potassium channels regulate heat production in brown adipose tissue.
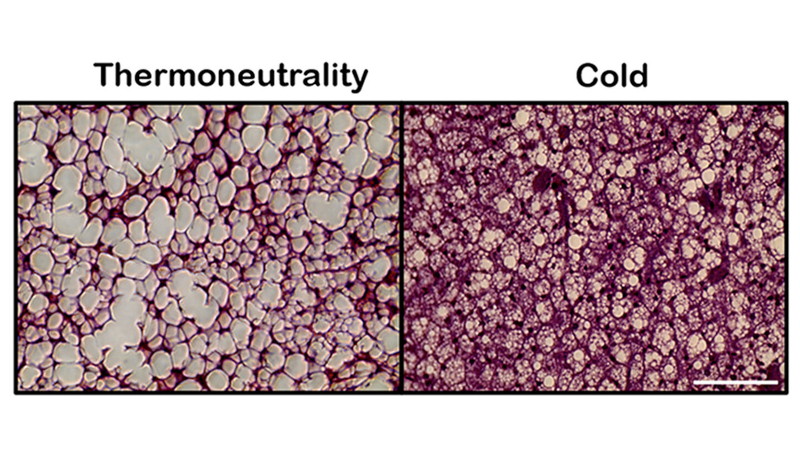
Sections of brown adipose tissue from mice kept at thermoneutrality or cold for 14 days, revealing tissue remodeling at different temperatures (image: Osvaldo Rodrigues Pereira Júnior et al./American Journal of Physiology-Cell Physiology)
Agência FAPESP* – A study conducted by researchers at the Center for Redox Processes in Biomedicine (Redoxoma) has shown that ATP-sensitive mitochondrial potassium channels (MitoKATP) are involved in both the development of brown fat cells and the activation of mitochondrial uncoupling in these cells, a process that dissipates energy in the form of heat.
Redoxoma is a Research, Innovation, and Dissemination Center (RIDC) funded by FAPESP and based at the University of São Paulo’s Institute of Chemistry (IQ-USP) in Brazil.
“We discovered that for brown fat cells to produce heat efficiently, the mitochondrial potassium channel must be closed,” explains Osvaldo Rodrigues Pereira Júnior, a researcher at Redoxoma. “This is the first time this phenomenon has been reported, revealing a previously unknown component of thermogenic activation.”
Brown adipose tissue, also known as brown fat, is one of the main factors responsible for maintaining body temperature in mammals by producing heat through a process called non-shivering thermogenesis. It consumes large amounts of energy to do this, making it a promising target for therapeutic strategies against obesity. Understanding how brown fat is activated may pave the way for new ways to regulate energy expenditure and promote metabolic health.
Pereira Júnior developed the study during his master’s degree at the Energy Metabolism Laboratory at IQ-USP with a scholarship from FAPESP, under the guidance of Professor Alicia Kowaltowski of IQ-USP.
The research was published in an article in the American Journal of Physiology-Cell Physiology.
Thermogenic activation
The study showed that, in mice, exposure to cold and adrenergic stimulation, i.e., the action of hormones typical of the response to cold and stress, modulates the levels of the MitoKATP channel in brown adipose tissue.
To better understand the function of this channel, the researchers removed the gene that encodes an essential subunit of MitoKATP in human preadipocytes, which are precursor cells of adipose tissue. They observed a decrease in oxygen consumption, reduced cell proliferation, and difficulty in the differentiation of these precursors into mature adipocytes. In mouse cell lines, the absence of the same protein compromised cellular respiration in the precursor stage but not in already differentiated cells.
The most surprising data came from mature adipocytes, however. The researchers observed an increase in oxygen consumption when they inhibited the MitoKATP channel, suggesting that closing the channel is necessary for brown adipose tissue thermogenesis to reach maximum efficiency.
This observation was confirmed in mitochondria isolated from mice treated with a compound that activates specific adrenergic receptors in brown fat. Under these conditions, inhibiting MitoKATP also increased oxygen consumption linked to thermogenesis.
According to Kowaltowski, the study’s results provide two pieces of complementary evidence for the importance of closing the MitoKATP channel in activating thermogenesis. “In addition to observing that cells with the channel closed generate more heat, we also saw that in animals in a condition that stimulates heat production, the channels were more closed,” the professor comments.
She points out that these two findings converge on the same conclusion: “At the same time that the tissue is being activated to generate heat, the channel closes. This indicates that there’s some type of signaling within the cell that leads to this ideal situation for heat generation. These are two different ways that demonstrate the importance of closing this channel to generate maximum heat.”
The article “Mitochondrial ATP-Sensitive K+ Channels (MitoKATP) Regulate Brown Adipocyte Differentiation and Metabolism” can be read at journals.physiology.org/doi/full/10.1152/ajpcell.00070.2025.
* With information from Maria Celia Wider from Redoxoma.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





