

Histological slides showing regions of the brain with necrosis in unvaccinated animals infected with the Zika virus (image: Nelson Côrtes et al./NPJ Vaccines)
The vaccine is being developed by researchers at the University of São Paulo and is based on technology known as “virus-like particles” (VLPs), which does not use genetic material from the pathogen.
The vaccine is being developed by researchers at the University of São Paulo and is based on technology known as “virus-like particles” (VLPs), which does not use genetic material from the pathogen.
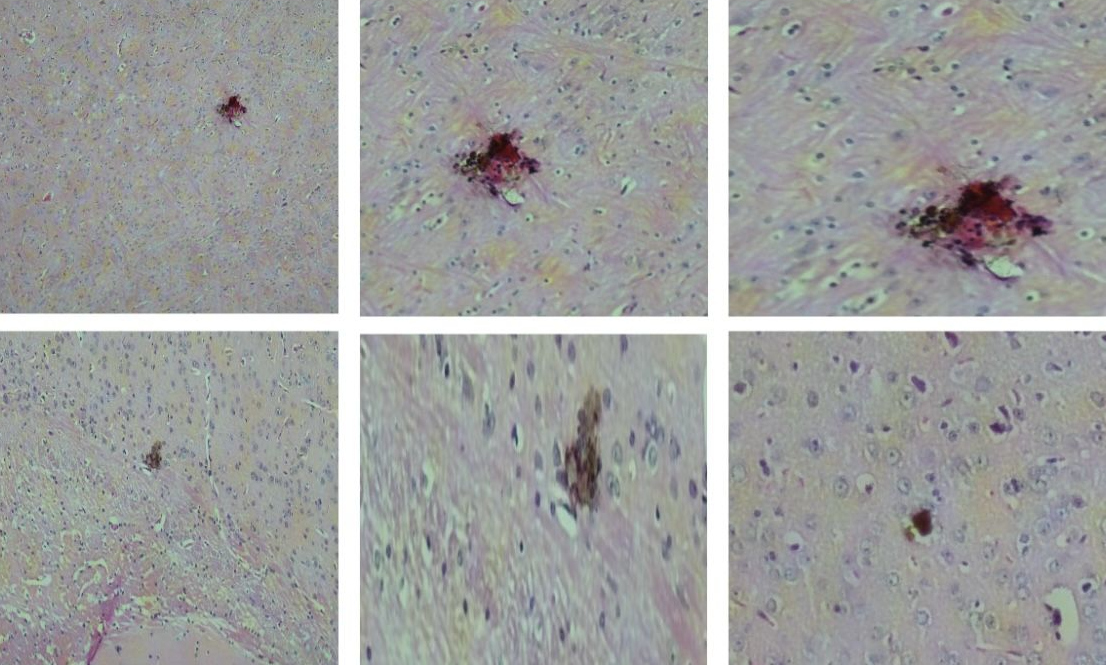
Histological slides showing regions of the brain with necrosis in unvaccinated animals infected with the Zika virus (image: Nelson Côrtes et al./NPJ Vaccines)
By Maria Fernanda Ziegler | Agência FAPESP – A new Zika virus vaccine developed in Brazil by researchers at the Institute of Tropical Medicine of the University of São Paulo’s Medical School (IMT-FM-USP) has been shown to be safe and effective in tests with mice. In addition to inducing an immune response against the pathogen, the vaccine also protected the animals from the brain and testicular damage typically caused by viral infection. The findings were published in the scientific journal NPJ Vaccines and represent a significant advancement in Zika prevention strategies. The research received funding from FAPESP.
“It’s been ten years since the Zika epidemic began in Brazil, and the disease continues to be a threat to public health, especially for pregnant women and their babies. In the study, we were able to design a formulation that can neutralize the pathogen and protect rodents from both brain inflammation, one of the most worrying consequences of infection, and testicular damage, something that hasn’t been observed in epidemiological studies, but is a notable feature of the disease when studied in the laboratory,” explains Gustavo Cabral de Miranda, a researcher supported by FAPESP who is responsible for the project.
Miranda explains that the formulation uses a strategy based on a technology known as “virus-like particles” (VLPs). “Unlike more traditional strategies, which use attenuated or inactivated virus inoculation, in this formulation we don’t use the pathogen’s genetic material, which makes its development much safer and more economical without the need for substances that enhance the immune response [adjuvants],” the researcher explains.
He says the technology is essentially divided into two components: the carrier particle (VLP), which makes the immune system recognize the presence of a virus; and the viral antigen, which stimulates the immune system to produce specific antibodies – in this case against the Zika virus – that prevent the pathogen from entering cells.
For the vaccine developed by the IMT researchers, a well-studied platform called QβVLP was used as the VLP. This mimics the viral structure, enabling the immune system to “recognize” a threat. The chosen antigen was EDIII, which is part of the Zika virus envelope protein that connects to a receptor in human cells (read more at: agencia.fapesp.br/54042).
“We inoculated the VLPs produced in the USP laboratory using bacteria [Escherichia coli], chemically conjugated to the antigen. This combined structure mimics a real virus, with EDIII attached to the outside of the platform,” describes Nelson Côrtes, the first author of the study. “When the formulation is injected into the body, this combination activates a strong immune response, including antibodies and Th1 cells, a subtype of T lymphocytes that play crucial roles in the immune response.”
Tests conducted on genetically modified mice that are more susceptible to the virus showed that the vaccine induced the production of antibodies that neutralized the virus. These antibodies prevented the infection from worsening and, consequently, the onset of symptoms.
The researchers also investigated the effects of Zika virus infection on various organs in mice, such as the brain, kidneys, liver, ovaries, and testicles. “The vaccine demonstrated the ability to protect male mice against testicular damage,” Côrtes says. “This is important, given the known risks of sexual transmission of the Zika virus and its potential to cause testicular damage, which can negatively affect spermatogenesis and reproductive health as a whole,” the researcher emphasizes.
Calibrated aim
The Zika virus poses an additional challenge to vaccine development due to its similarity to the four serotypes of the dengue virus, which circulate in the same environment. This similarity means that antibodies can “confuse” one pathogen with another. This is what scientists call a cross-reaction. At first, this may seem beneficial because the immune system recognizes a similar virus.
However, if the antibodies are not strong enough to prevent a second infection by a different dengue serotype, for example, the result is a boomerang effect. The antibodies bind to the virus, making it easier for the host cell to engulf the pathogen. Thus, the body itself helps the pathogen infect cells.
“The vaccine doesn’t cause a cross-reaction, which is very positive. Previous studies by the group had already analyzed this issue, and using the EDIII antigen allows the immune system to produce antibodies that are more specific to the Zika virus, avoiding the problem,” Miranda says.
The article “A VLPs based vaccine protects against Zika virus infection and prevents cerebral and testicular damage” can be read at www.nature.com/articles/s41541-025-01163-4.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





