

The researchers set out to analyze the performance of amorphous nickel phosphide (Ni-P) electrodes synthesized via electrodeposition on nickel foam (image: CDMF)
The nickel phosphide electrode proved effective and efficient as a catalyst in processes designed to produce H2 via water molecule breakdown.
The nickel phosphide electrode proved effective and efficient as a catalyst in processes designed to produce H2 via water molecule breakdown.
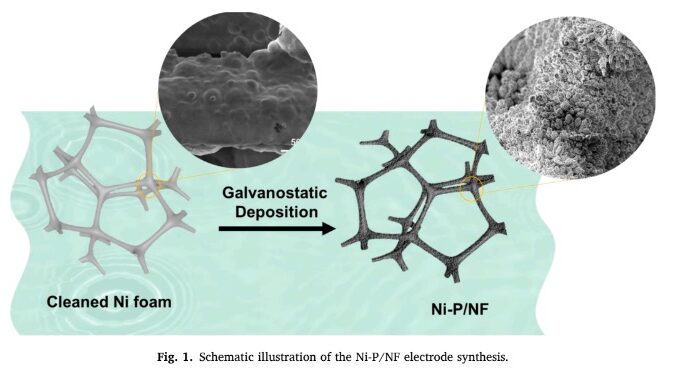
The researchers set out to analyze the performance of amorphous nickel phosphide (Ni-P) electrodes synthesized via electrodeposition on nickel foam (image: CDMF)
Agência FAPESP* – Hydrogen (H2) is considered a possible alternative to fossil fuels, which are responsible for a large proportion of atmospheric emissions and global warming, but production costs must be lowered if it is to become a viable option.
In an article published in the journal Electrochimica Acta, scientists at the Center for Development of Functional Materials (CDMF), a Research, Innovation and Dissemination Center (RIDC) funded by FAPESP and hosted at the Federal University of São Carlos (UFSCar) in São Paulo state, Brazil, describe the synthesis of a nickel phosphide electrode that showed high efficiency in hydrogen evolution reaction (HER) electrocatalysis. This type of reaction, which is still costly, breaks down water molecules to release hydrogen ions in a process known as hydrolysis.
Electrochemical production of hydrogen by hydrolysis is a promising technique with zero carbon emissions. Its efficiency depends on the capacity of the electrocatalyst.
In the article, the researchers describe an experiment designed to analyze the performance of amorphous nickel phosphide (Ni-P) electrodes synthesized via electrodeposition on Ni foam used as an HER electrocatalyst. The 3-Ni-P electrode performed outstandingly in alkaline, neutral and acidic conditions. The Ni-P films showed excellent stability under the different conditions studied.
The electrode’s strong performance was attributed to its granular structure, with a large surface area enabling good interaction with the electrolyte and endorsing HER kinetics. According to the authors, the results are relevant to the search for a catalyst that is stable, easy to synthesize, and capable of operating in a wide range of pH with high efficiency for the production of hydrogen from water.
The first author of the article is Lucia Mascaro. The other co-authors are Anelisse Brunca da Silva, Marina Medina, and Lorena Goulart.
The article “One-step electrodeposited nickel phosphide electrode for pH-universal electrochemical hydrogen production” is at: www.sciencedirect.com/science/article/abs/pii/S0013468623018479.
* With information from CDMF.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





