

Image: Miguel Tedde
The device was developed by researchers at the University of São Paulo’s Heart Institute (INCOR) and a Brazilian company with FAPESP’s support. It is biocompatible and offers other advantages over the imported product used hitherto.
The device was developed by researchers at the University of São Paulo’s Heart Institute (INCOR) and a Brazilian company with FAPESP’s support. It is biocompatible and offers other advantages over the imported product used hitherto.
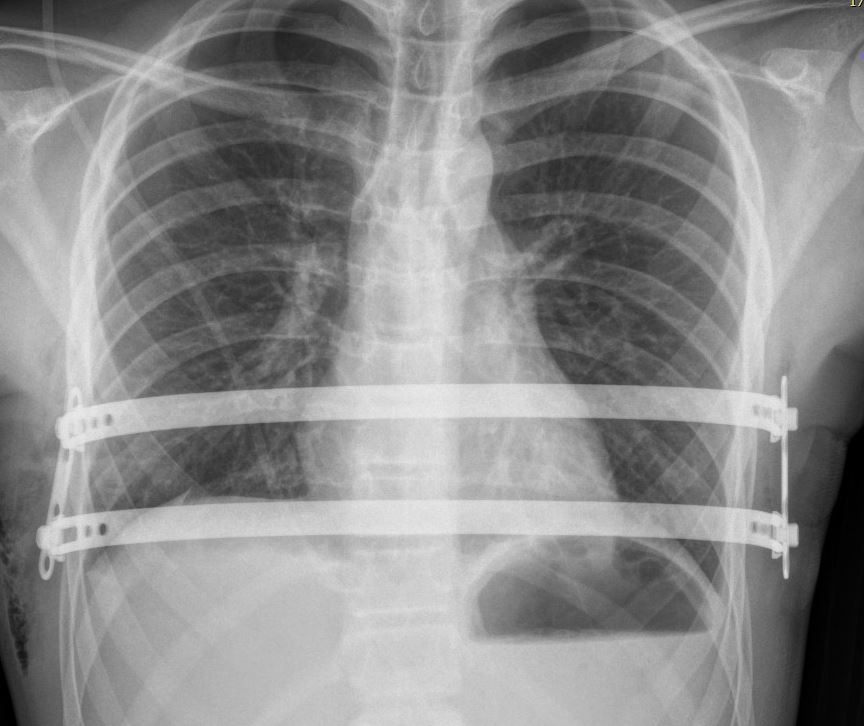
Image: Miguel Tedde
By Roseli Andrion | Agência FAPESP – In his day-to-day routine at the Heart Institute (INCOR) run by the University of São Paulo’s Medical School (FM-USP) in Brazil, thoracic surgeon Miguel Tedde now and again sees patients with a rare disorder called pectus excavatum, also known as sunken chest, in which the breastbone and ribs grow inward, creating a depression in the chest wall. Even mild cases can cause mental distress due to self-image issues. Severe cases may involve compression of the heart and lungs by the breastbone. The deformity can be treated surgically by placing an implant in the chest cavity to push the sternum forward. The implant is imported and made of steel, which is not biocompatible. Worse, the device may eventually become displaced.
“We’ve used the imported material in pectus excavatum repair surgeries for 20 years, and the results aren’t always adequate,” Tedde said.
To surmount these limitations, he has partnered with engineers at Traumec, an orthopedics firm based in Rio Claro (São Paulo state), to develop an entirely indigenous prosthesis with several advantages compared to the imported device. The project was supported by FAPESP.
The prosthesis has been successfully implanted at INCOR in 50 patients diagnosed with pectus excavatum. It has also been used in a minimally invasive procedure (the first ever, as far as Tedde is aware) to repair pectus carinatum (pigeon chest), in which the breastbone and ribs protrude anteriorly. It has been registered with ANVISA, the national health surveillance agency, which regulates drugs and medical procedures, and has been used in operations performed at other hospitals in Brazil.
“Brazilian surgeons now have at their disposal the most complete material in the world to perform the minimally invasive procedure for repair of the deformity. With this prosthetic device, there’s less risk of an operating accident, displacement doesn’t occur, and the anatomical results are better,” Tedde said.
The prevalence of pectus excavatum in Brazil is 1.2%. The condition is estimated to occur in one in 200 live births. It affects both sexes, but males are more likely to seek treatment. In women, the breasts may disguise the deformity, Tedde said, adding that “they may not seek treatment as often for that reason”.
The condition is due to abnormal growth of the cartilage that attaches the sternum to the ribs. The concavity may cause compression of the heart and lungs, but the psychological effects are particularly troublesome. “Patients are usually in the personality formation stage. Healthy boys and men enjoy exposing their chest, but those with the malformation are ashamed to do so. Not treating the disorder isn’t an option,” Tedde said.
In some cases, the condition is diagnosed at birth. In others, it becomes evident later. “Many patients have a family history of the disorder. That doesn’t mean a child will necessarily have it if a parent does, but patients frequently recall that their father or uncle or other relative has it,” he explained.
The only treatment is surgery, involving a procedure to implant a molded metal prosthesis behind the sternum so as to push it forward. Before the locally produced implant was developed, surgeons used an imported stainless steel device with serrated edges and two stabilizer bars, but these were not always effective and the bars could move, compressing mediastinal structures such as the heart and large blood vessels. “This displacement is dangerous,” Tedde said.
Advantages of the indigenous device
The Brazilian implant, which has been patented, is made of titanium, an element that is chemically and biologically more compatible with the human body, reducing the occurrence of allergic reactions. The bars have smooth extremities, and the stabilizers are oblique with pressure screws for anchoring. “I combined good ideas with solutions that had already been validated to create a better device that makes the procedure safer,” Tedde said.
In the initial project, 30 patients were operated on without complications. Because there was still material to be developed, another group of 20 patients were treated with the technique. “As a physician, I detected a clinical problem that called for repair. I’m particularly interested in identifying situations that aren’t ideal, and in developing solutions. This malformation is neglected because there aren’t millions of patients, and so there’s a risk of lack of interest in finding solutions,” he said.
The repair can be performed on patients of almost any age. South Korean surgeon Hyung-Joo Park, one of the world’s leading experts on pectus excavatum surgery, has treated patients aged from 3 to 55. “The age bracket is wide,” said Tedde, who has operated on patients aged from 9 to 42. “Most patients are between 12 and 17, however.”
The process entails modeling of cartilage rather than bone. Sternum joints typically close up as the individual grows, and the thoracic canal is fully closed at around age 35. “After that, there’s practically no change in bone shape, but cartilage shape can change, so expectations for anatomical results in older patients should be lower,” Tedde said.
The procedure is minimally invasive thanks to video-assisted thoracoscopy, with a lateral incision (formerly frontal and then median). The length of hospital stay has been reduced thanks to the Brazilian implant, falling from seven to five days for the first 30 patients in the project, and to four days for the next 20.
The prosthesis is removed three years after the operation. “It’s hard to do CPR [compressions] for cardiac arrest if the patient has a metal device in their chest, so it stays only long enough to fulfill its function,” Tedde explains. All patients treated during the research project have had the prosthesis removed, and no relapses have been observed in any of them. “We believe the condition doesn’t return, and this is confirmed by the literature. After the device is removed, there may be a slight caving-in, but this is less than 1 cm and is more an accommodation than a relapse.”
The procedure itself has changed, he continued. The implant used to be inserted from right to left, with a high risk of injuring the heart. More than 26 cardiac lesions are described in the literature. “I therefore decided to insert it from left to right, so that I had a good view of the heart as I passed the device through, making the procedure safer. I performed it in this manner on 28 patients. Also, we now use a crane to elevate the sternum,” he said.
Entirely local R&D
Unlike research on diseases such as cancer, diabetes or cardiopathy, for example, all the research involved in development of the prosthesis was conducted in Brazil. “These other research fields incur heavy costs. Most studies on such diseases are conducted abroad and only recruit patients in Brazil,” Tedde said.
The project covered the complete R&D cycle. “We began with the clinical problem, explored the science and ended with a Brazilian product for patient care. That’s what a complete R&D cycle should be like. The indigenous device is now competing with imported products. We’re very proud of the achievement,” Tedde said.
Sabará Hospital in São Paulo city, the largest children’s hospital in Brazil, is one of the project’s most recent partners. “They didn’t treat the condition but opened an outpatient clinic and the repair procedure will now be part of their pediatricians’ day-to-day work,” Tedde said, explaining that the hospital’s surgeons were reluctant to perform the previous repair operation because it was “very complicated. We haven’t eliminated risk completely, but it’s now much safer and the result is more effective than before”.
Only physicians and surgeons who are closely involved with this type of procedure are aware of the innovation. “Those who don’t know the device may have the impression it’s just a new material and may not know how much the surgical procedure has improved. Publicity is therefore important. Colleagues and patients need to know the procedure has changed for the better,” Tedde said.
Pigeon chest
Another condition that can be treated with the prosthesis is pectus carinatum, often referred to as pigeon chest, with a prevalence of 0.6%. In this case, the breastbone and ribs push outward, and ideally the treatment should use a chest compressor to reverse the deformity. If this does not work, surgery is performed. Use of the compressor is often avoided in children, however.
Tedde is now using the prosthetic device to repair pectus carinatum, inserting one bar over the sternum for compression and two under it for support. He calls this his sandwich method. He has used it once so far. “As far as we’re aware, this is the first case of pectus carinatum repaired by a minimally invasive procedure in Brazil,” he said.
Tedde will now submit a research project on treatment of this condition. “We’ll use the same material. The manufacturer has applied to ANVISA for permission to use it in pectus carinatum repair,” he said.
He also believes the material could be exported. “It needs to mature, of course. The logistics of marketing and distribution could be a difficulty. From the standpoint of product quality, however, it’s absolutely ready,” he said.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





