

Upper image: Phrixotrix hirtus railroad worm; lower image: red and far-red bioluminescence of E. coli expressing P. hirtus’s luciferase in presence of luciferin analogs D-luciferin (A), 6'-morpholinoluciferin (B), and 6'-pyrrolidinylluciferin (image: Scientific Reports)
Color differences in the light produced by the larviform beetle are known to be caused by two enzymes with minor structural differences, but the details were hitherto unknown. This discovery has potential for applications in biotechnology.
Color differences in the light produced by the larviform beetle are known to be caused by two enzymes with minor structural differences, but the details were hitherto unknown. This discovery has potential for applications in biotechnology.
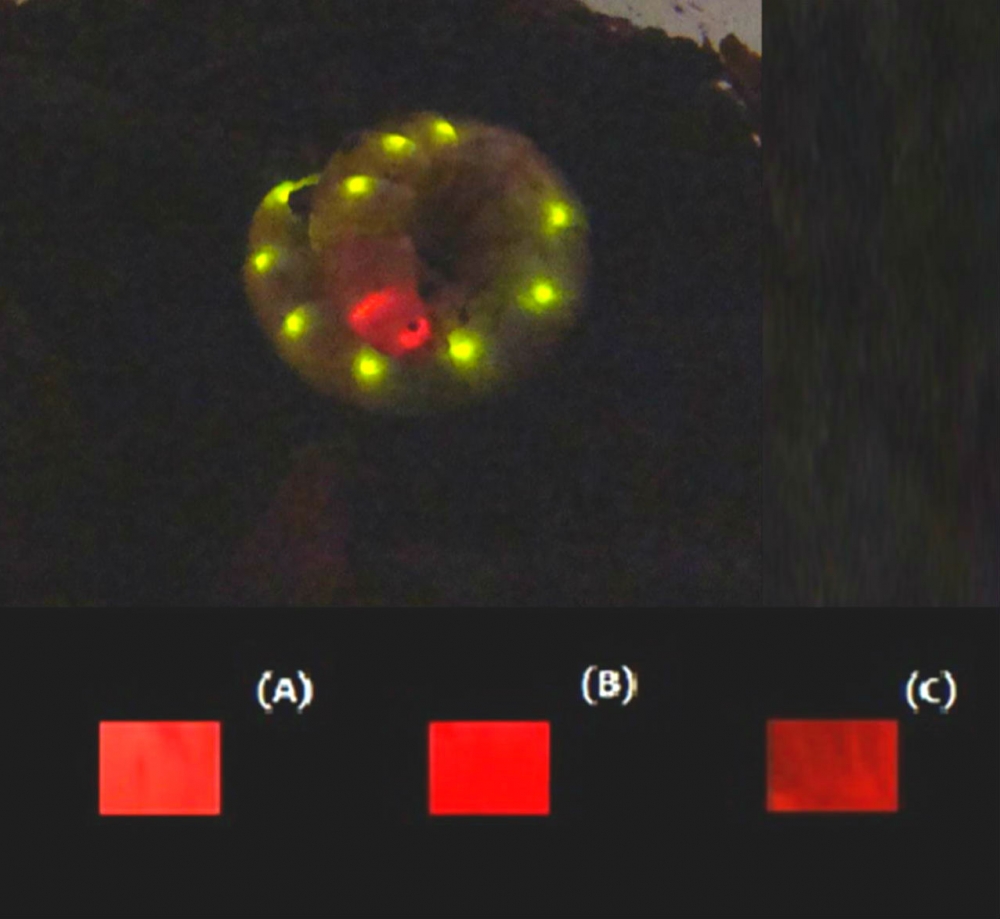
Upper image: Phrixotrix hirtus railroad worm; lower image: red and far-red bioluminescence of E. coli expressing P. hirtus’s luciferase in presence of luciferin analogs D-luciferin (A), 6'-morpholinoluciferin (B), and 6'-pyrrolidinylluciferin (image: Scientific Reports)
By André Julião | Agência FAPESP – One research group comprising Brazilian and Japanese scientists have discovered how luciferase produced by the railroad worm Phrixothrix hirtus emits red light.
Luciferase is an enzyme that catalyzes the oxidation of luciferin in fireflies, producing oxyluciferin and enabling fireflies to emit light. Differences in the molecular structures explain the different colors of this bioluminescence in different species. This discovery has the potential for new biotechnological applications, such as the imaging of muscles, blood and hemoglobin-rich tissue.
An article published in Scientific Reports describes the study, which was conducted by researchers at the Federal University of São Carlos (UFSCar) in São Paulo State, the National Bioscience Laboratory (LNBio) attached to Brazil’s National Energy and Materials Research Center (CNPEM), and the University of Electro-Communications in Tokyo, Japan.
The Brazilian team used cloned railroad worm luciferase, which naturally emits red light, and mutants of the enzyme together with a larger analog of luciferin synthesized by the Japanese team.
“This novel combination of luciferase with a luciferin analog not only revealed the larger size of the cavity in the luciferase but also produced far-red light more efficiently and is ideal for biomedical applications involving imaging of cells and tissues that preferably absorb blue-green light, such as mammalian cells,” Vadim Viviani, a professor at UFSCar (Sorocaba campus) and principal investigator for the study, told Agência FAPESP.
In a previous study, the group led by Viviani showed that luciferase from fireflies, which are close relatives of railroad worms, changed the color of the light they emitted from green to red in a test tube in response to a change in the acidity of the medium or the presence of heavy metals (read more at: revistapesquisa.fapesp.br/en/2019/06/24/green-yellow-or-red/).
The group did not yet know how red light was produced naturally by railroad worm luciferase, however, and has now shown how the phenomenon occurs in this species of beetle. Vanessa Rezende Bevilaqua, the first author of the article, participated in the research for her PhD with FAPESP’s support.
The study was also part of the FAPESP Thematic Project “Arthropod bioluminescence”.
P. hirtus is native to the Americas and is one of the few animals known to emit red light as well as green-yellow light, which is more common. During the larval stage, P. hirtus has several green “lanterns” on its back and a red one on its head. The latter helps the beetle find its way in the dark. The light sources on its back serve to frighten predators.
When males of the species become adults, they lose the red lantern but keep the two green ones. Adult females keep them all.
“We’ve now shown that yellow-green luciferases have a smaller cavity at the active site where luciferin binds and is oxidized to oxyluciferin,” Viviani said. “The luciferin is compressed into a more rigid environment, leading to electrostatic repulsion between the two molecules [the energized oxyluciferin and the walls of the luciferase active site], releasing light that contains more energy and is therefore green or yellow.”
In the case of the red luciferase produced by the head, the active site cavity is larger, there are more water molecules present, and the environment is less rigid, leading to a reduction in the electrostatic repulsion between the luciferin and the walls of the luciferase active site. This is why the light emitted is red, which contains less energy.
Bioimaging
To investigate the interactions that lead to the emission of red light, researchers have learned over the past few decades to clone various luciferases by using genetic engineering tools to modify some amino acids.
The Japanese group, led by Takashi Hirano at the University of Electro-Communications, synthesized red light-emitting luciferin analogs, which were tested by Bevilaqua with the firefly and railroad worm luciferases cloned and modified by the Brazilian research group.
Some of these modified luciferins had a larger structure than the others, and these larger luciferins interacted best with railroad worm luciferase, emitting far-red light more efficiently, whereas they did not interact efficiently with the green or yellow luciferases.
“The luciferases that catalyze green and yellow light have a small cavity and therefore don’t bind well to the large-structure luciferin analogs, which have very little luminescent activity,” Viviani said. “On the other hand, these large analogs interact well with luciferases that catalyze red light. We deduced from this that railroad worm luciferase has a large active site cavity capable of binding to the analogs.”
Having achieved this result, the researchers began testing new combinations of luciferins with modified railroad worm luciferase, eventually creating a more intense red light than that produced by P. hirtus. They believe that the combinations could be used in biomedical research.
“The luciferin analogs synthesized by the Japanese group aren’t the first to be created but offer the advantage of having more luminescent activity and a red-shifted light spectrum when they’re combined specifically with luciferase from P. hirtus. The commercially available analogs are less efficient, albeit more red-shifted,” Viviani said.
Initially, the idea is that this discovery could be used to enhance the visualization of biochemical and cellular processes in mammalian substances that do not absorb red light, such as blood cells and muscle tissue.
“When these substances are examined with conventional luciferase that emits green, yellow or blue light, it’s impossible to see biochemical and pathological processes clearly because pigments such as hemoglobin and myoglobin absorb most of the light in these parts of the chromatic spectrum,” Viviani said.
The article “Phrixotrix luciferase and 6′-aminoluciferins reveal a larger luciferin phenolate binding site and provide novel far-red combinations for bioimaging purposes” (doi: 10.1038/s41598-019-44534-3) by Vanessa R. Bevilaqua, T. Matsuhashi, G. Oliveira, P. S. L. Oliveira, T. Hirano and Vadim R. Viviani can be read at: www.nature.com/articles/s41598-019-44534-3.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





