

Technique developed in Brazil uses infrared light to release photoactive material and cancer drug inside tumor cells and kill them with hyperthermia (image: GNano-IFSC-USP)
Technique developed in Brazil uses infrared light to release photoactive material and cancer drug inside tumor cells and kill them with hyperthermia.
Technique developed in Brazil uses infrared light to release photoactive material and cancer drug inside tumor cells and kill them with hyperthermia.
Technique developed in Brazil uses infrared light to release photoactive material and cancer drug inside tumor cells and kill them with hyperthermia (image: GNano-IFSC-USP)
By Elton Alisson in São Carlos (Brazil) | Agência FAPESP – An optimized method to treat tumors by photothermal therapy, involving the conversion of light into heat, has been developed in Brazil by researchers in the Nanomedicine and Nanotoxicology Group (GNano) at the University of São Paulo’s São Carlos Institute of Physics (IFSC-USP).
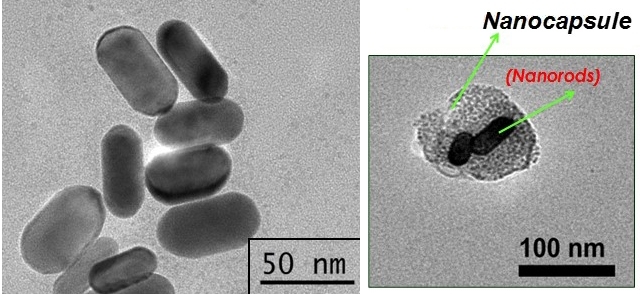
The technique consists of using nanocapsules made from cancer cell membranes to deliver an anticancer drug and photoactive material into a tumor. When the nanocapsules are irradiated with infrared light, they burst and release the photoactive material, which converts the light (optical energy) into heat (thermal energy), exposing the tumor cells to high temperatures and killing them with hyperthermia.
Nanocapsules are typically between 20 billionths and 300 billionths of a meter in size.
The method was developed as part of the PhD research of Valéria Spolon Marangoni, supported by a scholarship from FAPESP. The results of its use to treat bladder cancer in mice were described in a presentation to the first symposium on research and innovation in functional materials held by the Center for Development of Functional Materials (CDMF) on May 23-24, 2019, at the Federal University of São Carlos (UFSCar).
CDMF is a Research, Innovation and Dissemination Center (RIDC) funded by FAPESP.
“We developed a nanocarrier that could be a candidate to improve the transport, release and activation of drugs used in photothermal cancer therapy,” Valtencir Zucolotto, Full Professor at IFSC-USP and principal investigator for the study, said during the event.
The novel system used nanoparticles made of theranostic materials developed by the team in recent years. Theranostics (therapeutics + diagnostics) is an emerging field in which drugs and techniques are combined to diagnose and treat medical conditions simultaneously.
When the nanoparticles are introduced into the bloodstream, they seek out and enter tumor cells while their movement through the organism is tracked by tomography, magnetic resonance or photoacoustic spectroscopy, for example.
Once the nanoparticles are visualized, they can be heated by magnetism – if they contain a magnetic material such as magnetite – or photothermally to kill the tumor cells with hyperthermia.
“When irradiated with infrared light, graphene oxide nanoparticles incubated in HeLa cells, for example, heat the cells by 8 to 12 degrees, and this heating kills the cells,” Zucolotto said. HeLa cells are laboratory-grown human cancer cells that are naturally immortal, meaning they do not die after a set number of cell divisions.
Gold nanorods
In addition to graphene, researchers have used gold to create theranostic nanoparticles in star and rod shapes, which make nanomaterials capable of absorbing infrared light and converting it into thermal energy, the researchers explained.
Spherical gold nanoparticles are effective carriers for use in drug delivery systems but absorb only visible light. “This prevents their use in photothermal therapy because visible light doesn’t penetrate tissue well, unlike infrared,” Zucolotto said.
Materials engineers recently discovered that if spherical gold nanoparticles are stretched into a rod-like shape, they become capable of longitudinal electronic vibration and infrared light absorption.
Inspired by this discovery, the IFSC-USP team of researchers began producing gold nanorods and testing their use in photothermal therapy for some types of cancer.
To transport the requisite compounds into target cells, they developed nanocapsules made from cultured lung cancer cell membranes.
Most nanocapsules used to deliver drugs and other molecules to specific regions of an organism or target cells are currently made from lipids and polymers.
According to Zucolotto, cell membrane nanocapsules are more efficient carriers because they are made of the same material as the target cells.
“The close resemblance between the composition of cell membrane nanocapsules and that of tumor cells, with proteins such as galectins, facilitates mutual recognition and adhesion. As a result, the nanocapsules interact more with the target cells and can more efficiently deliver the material they carry,” he said.
By constantly refining the technique for obtaining these nanocapsules, the researchers have succeeded in inserting larger amounts of gold nanorods and cancer drugs into them.
In a recent study published in the journal Applied Bio Materials, they used gold nanorods and the chemotherapy drug beta-lapachone inserted into cell membrane nanocapsules to treat bladder tumors induced in mice.
The results of the experiments, which were conducted in collaboration with Wagner José Fávaro, a professor at the University of Campinas’s Biology Institute (IB-UNICAMP) in São Paulo State, showed that the nanocapsules entered the tumor cells, burst when irradiated with infrared light for two minutes, and released the gold nanorods and beta-lapachone between 10 and 20 minutes after the start of the procedure.
Tissue analysis showed that none of the bladder tumors grew and that some even receded.
“We concluded that this method of treatment destroyed cancer cells by synergy between hyperthermia and chemotherapy,” Zucolotto said.
The Applied Bio Materials article “Photothermia and activated drug release of natural cell membrane coated plasmonic gold nanorods and β-lapachone” (DOI: 10.1021/acsabm.8b00603) by Valeria S. Marangoni, Juliana Cancino Bernardi, Ianny B. Reis, Wagner J. Fávaro and Valtencir Zucolotto can be read at pubs.acs.org/doi/10.1021/acsabm.8b00603.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





