

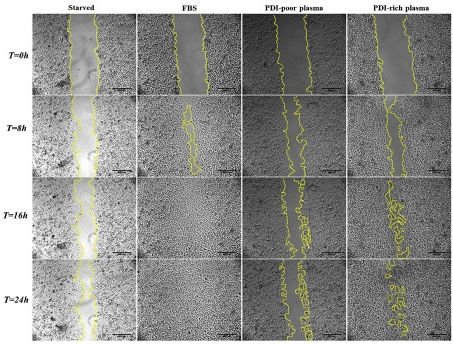
Study suggests that people with low levels of PDIA1 in blood plasma may be at high risk of thrombosis; this group also investigated PDIA1’s specific interactions in cancer (image comparing the migration of Human umbilical vein endothelial cells (HUVECs) cultured in PDI-poor plasma and of HUVECs cultured in PDI-rich plasma into the denuded area created by a pipette tip-inflicted wound (yellow line) within a 24 hour period / Redox Biology)
Study suggests that people with low levels of PDIA1 in blood plasma may be at high risk of thrombosis; this group also investigated PDIA1’s specific interactions in cancer.
Study suggests that people with low levels of PDIA1 in blood plasma may be at high risk of thrombosis; this group also investigated PDIA1’s specific interactions in cancer.

Study suggests that people with low levels of PDIA1 in blood plasma may be at high risk of thrombosis; this group also investigated PDIA1’s specific interactions in cancer (image comparing the migration of Human umbilical vein endothelial cells (HUVECs) cultured in PDI-poor plasma and of HUVECs cultured in PDI-rich plasma into the denuded area created by a pipette tip-inflicted wound (yellow line) within a 24 hour period / Redox Biology)
By André Julião | Agência FAPESP – Measuring the blood plasma levels of an enzyme called PDIA1 could one day become a method of diagnosing a person’s predisposition to cardiovascular disease even if they are healthy, i.e., not obese, diabetic or a smoker, and with normal cholesterol.
This is suggested by a study published in the journal Redox Biology by Brazilian researchers affiliated with the University of São Paulo (USP), the University of Campinas (UNICAMP) and Butantan Institute.
The investigation was conducted under the aegis of the Center for Research on Redox Processes in Biomedicine (Redoxome), one of the Research, Innovation and Dissemination Centers (RIDCs) funded by FAPESP. Redoxome is hosted by USP’s Chemistry Institute.
“This molecule belongs to the protein disulfide isomerase [PDI] family. Our study showed that people with low plasma levels of PDIA1 have a more inflammatory protein profile and hence run a higher risk of thrombosis. On the other hand, people with high levels of PDIA1 have more ‘housekeeping’ proteins associated with cell adhesion, homeostasis and the organism’s normal functioning,” said Francisco Rafael Martins Laurindo, a professor at the University of São Paulo’s Medical School (FM-USP) and principal investigator for the study.
The study was conducted during the PhD research of Percíllia Victória Santos de Oliveira with a scholarship from FAPESP.
The group analyzed blood plasma samples from 35 healthy volunteers with no history of chronic or acute disease. None was a smoker or a user of recreational drugs or chronic medication.
Plasma was collected 10-15 times at intervals of days or weeks during a period of 10-15 months. Circulating PDI levels were within a small range for most individuals. Moreover, in a cohort of five individuals, PDIA1 levels were measured three times in a nine-hour period. The variability of the results was again negligible.
“However, the measurements showed that some patients had high levels of PDIA1, while the levels were very low, almost undetectable, in others. When the tests were repeated for the same person over time, these values hardly varied at all,” said Laurindo, who heads the Translational Cardiovascular Biology Laboratory at the Heart Institute (InCor) attached to FM-USP’s teaching and general hospital (Hospital das Clínicas).
The researchers also measured the levels of PDIA1 in 90 plasma bank samples from patients with chronic cardiovascular disease. The analysis consistently showed low levels of the enzyme.
They then conducted several additional proteomic studies to investigate how the plasma levels of PDIA1 correlated with an individual’s protein signature. The adhesion and migration of cultured vein endothelial cells treated with PDIA1-poor plasma were impaired in comparison with those of cells treated with PDIA1-rich plasma.
These results led to the hypothesis that the plasma level of PDIA1 could be a window onto individual plasma protein signatures associated with endothelial function, which could indicate a possible predisposition to cardiovascular disease.
The study also showed no correlation between PDIA1 levels and well-known risk factors for cardiovascular disease, such as triglycerides and cholesterol.
The next steps for the research group include studying PDIA1 levels in conditions such as acute coronary disease, as well as other members of the protein disulfide isomerase family (there are more than 20 PDIs all told), to compare results and confirm whether all these enzymes are potential markers of vulnerability to cardiovascular disease.
Cancer marker
Another article by Laurindo and colleagues, published in the journal Cell Death & Disease, shows how PDIA1 regulates the production of reactive oxygen species, also known as free radicals. These species cause oxidative stress and can lead to cancer.
The study was part of the PhD research of Tiphany Coralie de Bessa at FM-USP, supported by a scholarship from FAPESP.
PDIA1 is a known marker of aggressiveness and resistance to chemotherapy in certain tumors. Previous papers by Laurindo et al. describe the role of this molecule in regulating blood cell Nox1, a major source of reactive oxygen species such as secondarily formed superoxide and peroxide.
In this new study, the researchers used colorectal tumor cells, which are known to overexpress Nox1. Three different strains were used. One of these strains (HCT116) carried a mutation of the gene KRas found in some 30% of colorectal, prostate and bladder tumors. Another strain (HKE3) had a weaker (less active) form of the mutation, while the third strain (Caco2) lacked the mutation and was used as a control.
The researchers detected overexpression of PDIA1 in both KRas-mutant tumor cell strains but particularly in HCT116. This might appear to be an advantage, as PDIs could help produce more superoxide and peroxide, which could potentially help combat the tumor.
“The problem was that when very high levels of superoxide were expressed in a tumor, the function of PDIA1 changed to one of limiting the production of reactive oxygen species and potentially protecting the tumor,” Bessa told Agência FAPESP.
Analysis of this limiting effect showed that it was caused by Rac1, a protein activated by mutant KRas.
Currently doing a postdoctoral research internship, Bessa is working on a more detailed characterization of the interaction between PDIA1 and NOX1 with a view to the future development of PDI inhibitors specific to the cancer context. They could be used, for example, in KRas-mutant tumors in parallel with chemotherapy to mitigate resistance to treatment.
Inhibitors
Clinical trials of inhibitors of other PDIs are being conducted by different groups of researchers in several parts of the world. Because these enzymes play various essential roles in cell survival, Laurindo explained, it is important to understand their specific interactions in the cancer context to design inhibitors capable of eliminating tumors with a minimum of toxicity to normal cells.
In another study, published in the American Journal of Physiology – Heart and Circulatory Physiology, the researchers used an antibody to inhibit PDIA1 on the surface of vascular cells and observed the effects of stimulation with several different mechanical forces, such as stretching and alterations to the rigidity of the extracellular matrix.
Resulting from research conducted during Leonardo Yuji Tanaka’s postdoctoral internship with support from FAPESP, the study concluded that surface PDIA1 inhibition affected the cytoskeleton, an intracellular framework of filaments, thereby hindering cell migration.
“PDIA1 is fundamental for the ability of cells to migrate within the organism, and so it mustn’t be completely inhibited. When the surface portion, which corresponds to less than 2% of total PDIA1, is silenced, the cell survives but loses fine regulation of cell direction during migration. This can be leveraged in the search for new disease mechanisms and drugs,” Laurindo explained.
The article “Protein disulfide isomerase plasma levels in healthy humans reveal proteomic signatures involved in contrasting endothelial phenotypes” (doi: 10.1016/j.redox.2019.101142) by Percíllia Victória Santos de Oliveira, Sheila Garcia-Rosa, Ana Teresa A. Sachetto, Ana Iochabel Soares Moretti, Victor Debbas, Tiphany Coralie De Bessa, Nathalia Tenguan Silva, Alexandre da Costa Pereira, Daniel Martins-de-Souza, Marcelo Larami Santoro and Francisco Rafael Martins Laurindo can be read at www.sciencedirect.com/science/article/pii/S2213231719300217.
The article “Subverted regulation of Nox1 NADPH oxidase-dependent oxidant generation by protein disulfide isomerase A1 in colon carcinoma cells with overactivated KRas” (doi: 10.1038/s41419-019-1402-y) by Tiphany Coralie De Bessa, Alessandra Pagano, Ana Iochabel Soares Moretti, Percíllia Victoria Santos Oliveira, Samir Andrade Mendonça, Herve Kovacic and Francisco Rafael Martins Laurindo can be read at www.nature.com/articles/s41419-019-1402-y.
The article “Peri/epicellular protein disulfide isomerase-A1 acts as an upstream organizer of cytoskeletal mechanoadaptation in vascular smooth muscle cells” (doi: 10.1152/ajpheart.00379.2018) by Leonardo Y. Tanaka, Thaís L. S. Araujo, Andres I. Rodriguez, Mariana S. Ferraz, Vitor B. Pelegati, Mauro C. C. Morais, Aline M. dos Santos, Carlos L. Cesar, Alexandre F. Ramos, Adriano M. Alencar and Francisco Rafael Martins Laurindo can be retrieved from www.physiology.org/doi/abs/10.1152/ajpheart.00379.2018.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





