

A long noncoding RNA protects cancer cells and increases their proliferation. The discovery could lead to treatment for drug-resistant types of melanoma (image: Enilza Espreafico & Douglas Elias Santos)
A long noncoding RNA protects cancer cells and increases their proliferation. The discovery could lead to treatment for drug-resistant types of melanoma.
A long noncoding RNA protects cancer cells and increases their proliferation. The discovery could lead to treatment for drug-resistant types of melanoma.
A long noncoding RNA protects cancer cells and increases their proliferation. The discovery could lead to treatment for drug-resistant types of melanoma (image: Enilza Espreafico & Douglas Elias Santos)
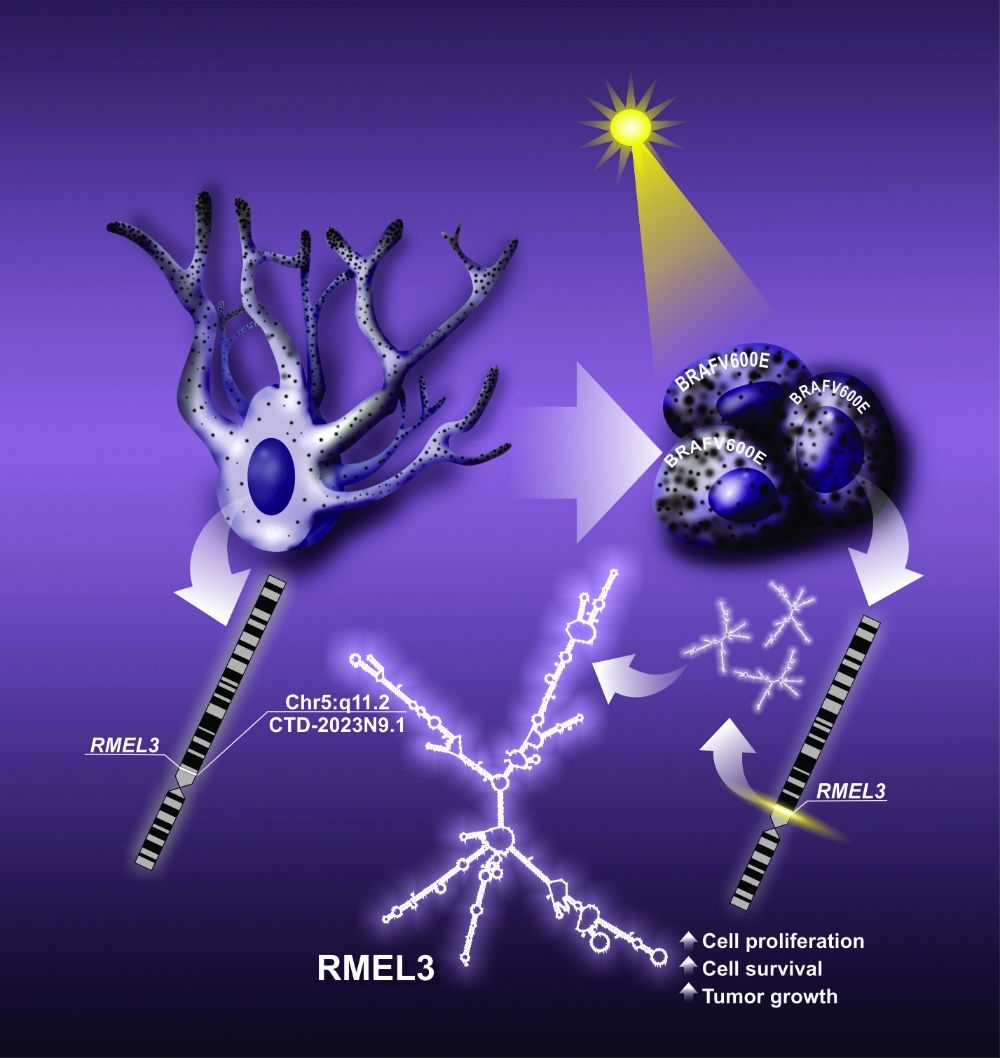
By André Julião | Agência FAPESP – New tests in animals and cultured melanoma cells have shown that RMEL3, a molecule present in most cases of this aggressive skin cancer, plays a key role in tumor survival and proliferation. The findings reinforce previous studies indicating that the inhibition of RMEL3 can be a novel therapeutic option where tumors are resistant to the most frequently used drug.
The research is supported by FAPESP. The latest results are published in the journal Pigment Cell & Melanoma Research.
“This research characterizes the role of a long noncoding RNA [that does not contain information for protein synthesis], RMEL3, in melanoma tumorigenesis. We found that when RMEL3 is introduced into nontumor cells [which normally do not express it], they can survive as if they were tumor cells: they no longer need growth factor stimulation from the extracellular medium,” said Enilza Espreafico, a professor in the University of São Paulo’s Ribeirão Preto Medical School (FMRP-USP) in Brazil and principal investigator for the study.
In another experiment, the group introduced RMEL3 into a melanoma cell line that normally expresses very low levels of this RNA. “The cells began proliferating more in culture, and when implanted in animals, they generated tumors that grew faster than tumors in the parent cells [without RMEL3],” Espreafico said.
The study was a collaboration with researchers at Harvard Medical School and the University of Texas in the United States and at other Brazilian institutions, including the Barretos Cancer Hospital, São Paulo State University’s Pharmaceutical Science School (FCFAR-UNESP) and the Center for Cell-Based Therapy (CTC). Hosted by FMRP-USP’s general hospital (Hospital das Clínicas), CTC is one of the Research, Innovation and Dissemination Centers (RIDCs) funded by FAPESP.
According to Espreafico, one of the drugs in current use to treat melanoma is vemurafenib, which directly inhibits mutated forms of BRAF, a protein present in most melanomas, but few patients benefit in the long term because melanoma is resistant to the drug. “We could create a molecule to silence RMEL3 and use it as a complementary treatment. This approach could attenuate tumor cell resistance to the drug,” she said.
RMEL3 could be a good alternative as a drug target, she added, because it is highly specific to melanoma and is found in hardly any healthy tissue. This characteristic suggests that its inhibition would not affect other kinds of tissue and would not have significant systemic effects.
“Expression in specific tissue and a specific type of cancer is a property of many long noncoding RNAs, making them excellent therapeutic targets,” she said.
Pioneering research
The study published in Pigment Cell & Melanoma Research is the third on the role of RMEL3 in melanoma conducted by the FMRP-USP group. Espreafico identified and named this RNA in an article published in 2010 after finding 29 candidates, including several potential long noncoding RNAs expressed only in melanoma cells.
Three drew attention because they were present in both cultured cells and tumor samples from patients. The inhibition of RMEL3 resulted in the largest number of cultured melanoma cell deaths, making it the preferred target in further research.
In the second study, completed in 2016, the group showed that inhibiting RMEL3 can reduce the viability of cultured melanoma cells by up to 95% and that this treatment has no effects on cells that do not express RMEL3. Many melanoma cases involve mutation of a gene called BRAF, and RMEL3 expression is normally high in these cases. The study showed that RMEL3 could be used to downregulate BRAF and other molecules involved in the control of cell proliferation and death (read more at: agencia.fapesp.br/23673).
The latest article by the group shows that the treatment of melanoma cells carrying the mutant BRAF with the drug vemurafenib inhibits the expression of RMEL3. This result means that RMEL3 expression in melanoma not only correlates with the presence of the BRAF mutation but is indeed dependent on hyperactivity of the mutated BRAF protein, which is key to melanoma tumorigenesis.
“RMEL3 must play an important role in this pathway since the induced expression of this molecule in cells that normally don’t express it promotes cell proliferation and survival even under conditions in which the cells are deprived of the presence of growth factors in the culture medium,” Espreafico said.
The group also analyzed the genome sequences available for melanoma in two large databases operated by The Cancer Genome Atlas Network (TCGA) and the International Cancer Genome Consortium (ICGC).
The datasets indicated a high frequency of mutations of the gene RMEL3 in melanomas, suggesting the influence of exposure to sunlight as the cause of the mutations. In addition, the researchers studied melanoma samples from 38 patients treated by the Barretos Cancer Hospital and found higher levels of RMEL3 in melanomas associated with long-term exposure of skin to sunlight.
In vivo tests
The researchers performed in vivo tests using immunosuppressed mice (to avoid rejection of human tissue) injected subcutaneously with melanoma cells that had been previously infected with a viral vector that carried the RMEL3 gene. The control group received cells injected only with an empty viral vector (without RMEL3).
Both groups received the same quantity of malignant cells. Tumors grew at a similar rate for the first fortnight, after which the tumors with RMEL3 grew more. At the end of the experiment, on the twenty-fourth day, they were significantly larger than those in the control group.
According to Espreafico, the results are promising and pave the way to search for a molecule capable of blocking RMEL3. This compound could be developed into a drug against melanoma, the most aggressive type of skin cancer. The researchers are currently analyzing the molecular mechanism of the action of RMEL3, an important step toward drug development and the design of clinical trials.
The article “The lncRNA RMEL3 protects immortalized cells from serum withdrawal-induced growth arrest and promotes melanoma cell proliferation and tumor growth” (doi: 10.1111/pcmr.12751) by Cibele Cardoso, Rodolfo B. Serafim, Akinori Kawakami, Cristiano Gonçalves Pereira, Jason Roszik, Valeria Valente, Vinicius L. Vazquez, David E. Fisher and Enilza M. Espreafico can be read at onlinelibrary.wiley.com/doi/full/10.1111/pcmr.12751.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





