

Researchers obtain sterilizing protection in murine assays performed with recombinant Plasmodium vivax protein (image: Frontiers in Immunology)
Researchers obtain sterilizing protection in murine assays performed with recombinant Plasmodium vivax protein.
Researchers obtain sterilizing protection in murine assays performed with recombinant Plasmodium vivax protein.
Researchers obtain sterilizing protection in murine assays performed with recombinant Plasmodium vivax protein (image: Frontiers in Immunology)
By Maria Fernanda Ziegler | Agência FAPESP – A new preclinical vaccine against Plasmodium vivax malaria, the most widespread form of the disease and the most prevalent in the Americas, has been tested in mice with 45% efficacy, an important advance in the development of prevention alternatives.
The P. vivax parasite was responsible for more than 13 million cases of malaria worldwide in 2015, according to the World Health Organization (WHO), and there are no vaccines against this pathogen.
The strategy behind the new vaccine is based on the development of recombinant versions of proteins found in the sporozoite, a form of the parasite present in the salivary glands of the mosquito, which transmits it to humans. The protein in question is homologous to another that is being used in a vaccine currently at a more advanced stage of development against P. falciparum, the most common malaria parasite on the African continent.
“Given the success of this protein from P. falciparum, we thought of trying something similar against the parasite that most infects people in the Americas. True, the protection in Africa isn’t high, ranging from 30% to 40%, but the vaccine has reduced the severe forms of P. falciparum malaria and delayed the first malaria episode in children, lowering child mortality,” said Irene Soares, a professor in the University of São Paulo’s School of Pharmaceutical Sciences (FCF-USP) in Brazil, and corresponding author of an article containing the results of this research published in the journal Frontiers in Immunology.
The candidate vaccine against P. falciparum, RTS,S/AS01, has passed Phase 3 clinical trials – clinical research is typically classified into four phases – and received WHO’s green light for a pilot phase in Africa. According to Soares, the vaccine will not be for general use in the population. “Perhaps for children and non-immune people such as travelers to the endemic region. It focuses on preventing severe forms of malaria,” she said.
The initiative to develop a vaccine against P. vivax was taken by a group of researchers at the Federal University of São Paulo’s Center for Cellular & Molecular Therapy and FCF-USP. The work is supported by FAPESP as part of a Thematic Project and is being conducted in collaboration with an international team from France’s Institut Pasteur and Singapore’s Agency for Science, Technology & Research (A*STAR), among others.
P. vivax has particular traits that hinder the development of vaccines against it. In contrast with that of P. falciparum, P. vivax’s target protein has three allelic variants, called VK210, VK247 and P. vivax-like. Prior research conducted in the 1990s and 2000s showed that all three variants were circulating in Brazil, with VK210 predominating.
The circumsporozoite protein (CSP), the most abundant component of the parasite’s surface, is well known to science. It was characterized in the 1960s by Brazilian researchers Ruth Nussenzweig and her husband Victor at New York University.
The molecule is involved in the initial stages of liver cell invasion in infected mammals. As a result, it is an important target for antibodies and other immune system cells.
“Because the P. vivax protein has three allelic variants, we produced a hybrid version combining all three. It contains a piece of each one. If the vaccine were based on a single variant, it wouldn’t protect people against the others and wouldn’t provide sufficient coverage,” Soares said.
After producing the fused protein, the researchers proceeded to the stage of inducing antibodies against the three variants. To find out whether the antibodies recognized the parasite, they turned for help to their collaborating colleagues at A*STAR.
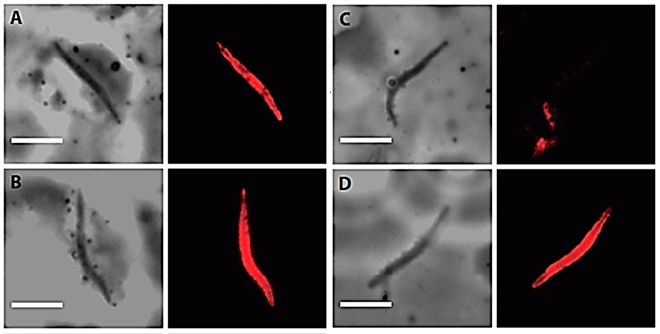
The Singapore agency provided sporozoites extracted from the mosquito’s saliva. Immunofluorescence assays showed that the antibodies generated by both the mixture of proteins and the fused protein were indeed able to recognize the native molecule.
Animal testing entailed another challenge: mice are not infected by P. vivax. To solve the problem, the team collaborated with colleagues at France’s Institute Pasteur, who tested a transgenic parasite – a sporozoite of P. berghei (which does infect mice) that expressed repeats of the P. vivax protein VK210 (Pb/PvVK210).
“This is why the article has several authors affiliated with different research centers. It was a highly complex study with a large number of stages,” Soares said.
At the laboratory headed by Rogerio Amino, a Brazilian researcher at Institut Pasteur in France and a co-author of the study, the vaccine protected mice against infection by chimeric P. berghei sporozoites expressing repeats of the P. vivax circumsporozoite protein.
“It was an important collaboration. The animals received three doses of the hybrid vaccine and were then challenged with the transgenic parasite,” Soares said. “Four of the six immunized mice remained uninfected until the tenth day after the challenge, whereas all non-immunized animals in the control group became infected after four days.”
Additional testing must be completed before the vaccine is proved commercially viable as an alternative against P. vivax, she added. For example, the vaccine must be tested in other mammals before embarking on the clinical trial stage.
The first author of the article, Alba Marina Gimenez from UNIFESP’s Center for Cellular & Molecular Therapy, recently visited Oxford University in the UK as a postdoctoral research intern and there was able to test transgenic parasites that expressed each of the three variants of the protein (the VK210, VK247 and P. vivax-like alleles). This research was supported by FAPESP.
Dormant parasites
Another major challenge for the development of a vaccine against P. vivax is the need to combat dormant parasites that remain in the liver after the acute phase is over and can trigger another malaria episode months after the patient was infected. This is another peculiarity of P. vivax.
“When the mosquito bites, some of the inoculated parasites assume a dormant form as hypnozoites in the liver, while others cause the disease,” Amino said. “Pathogens in the blood may be treated, but those that are ‘sleeping’ remain ready to attack again. The remedy may work to begin with, and then some months later the parasites may ‘wake up’ and return to the bloodstream, causing a relapse.”
Amino is also one of the authors of an article published in Trends in Parasitology, according to which a vaccine against P. vivax sporozoites with only moderate efficacy in combating primary infection can substantially reduce transmission of hypnozoites by preventing relapses.
“A vaccine that significantly reduced the number of hypnozoites would be worthwhile even if it weren’t 100% effective against primary infection,” Amino said. “The efficacy of the vaccine that’s being developed against P. vivax is good, but whether it can reduce relapses hasn’t been tested yet. If it also combats hypnozoites effectively, it will be a major advance.”
The Frontiers of Immunology article “Vaccine containing the three allelic variants of the Plasmodium vivax circumsporozoite antigen induces protection in mice after challenge with a transgenic rodent malaria parasite” (doi: 10.3389/fimmu.2017.01275) by Alba Marina Gimenez, Luciana Chagas Lima, Katia Sanches Françoso, Priscila M. A. Denapoli, Raquel Panatieri, Daniel Y. Bargieri, Jean-Michel Thiberge, Chiara Andolina, Francois Nosten, Laurent Renia, Ruth S. Nussenzweig, Victor Nussenzweig, Rogerio Amino, Mauricio M. Rodrigues and Irene S. Soares can be read at: frontiersin.org/articles/10.3389/fimmu.2017.01275/full.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





