
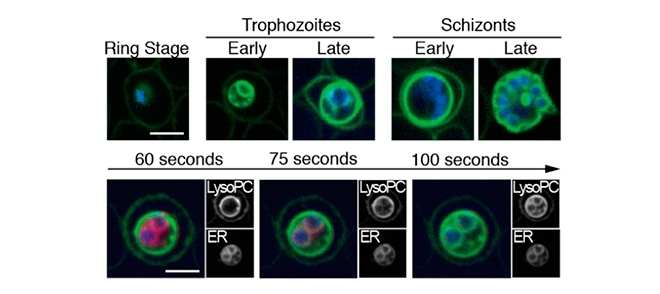
Study published in Cell shows that a drop in levels of a phospholipid in the human host’s blood signals to Plasmodium that it is time to change into the sexual form capable of infecting the vector mosquito (images: Marti, Clardy et al. / Cell)
Study published in Cell shows that a drop in levels of a phospholipid in the human host’s blood signals to Plasmodium that it is time to change into the sexual form capable of infecting the vector mosquito.
Study published in Cell shows that a drop in levels of a phospholipid in the human host’s blood signals to Plasmodium that it is time to change into the sexual form capable of infecting the vector mosquito.

Study published in Cell shows that a drop in levels of a phospholipid in the human host’s blood signals to Plasmodium that it is time to change into the sexual form capable of infecting the vector mosquito (images: Marti, Clardy et al. / Cell)
By Karina Toledo | Agência FAPESP – Researchers in the United Kingdom and the United States have begun to unpack the mechanisms by which the malaria parasite regulates a key stage in its life cycle: the moment when it stops reproducing in the human host’s blood cells – causing such symptoms as fever, aches and chills – and takes the first step in sexual differentiation by becoming a gametocyte, which is capable of infecting the vector mosquito.
According to a paper published in the journal Cell, falling levels of a phospholipid, called lysophosphatidylcholine, in human blood plasma appear to function as a signal that the time has come to “jump ship” and find another host.
The study described in the paper was presented at the University of São Paulo’s Pharmaceutical Science School (FCF-USP) by Matthias Marti, a professor of molecular parasitology at the University of Glasgow in Scotland. The presentation took place in March 2018 during the São Paulo School of Advanced Science in Cell Biology (SPCell), an event supported by FAPESP through its São Paulo School of Advanced Science Program.
“Understanding how the parasite transforms into a gametocyte can point to targets for the development of drugs that block transmission of the disease,” Marti told Agência FAPESP.
The results obtained so far are from in vitro experiments with protozoans of the species Plasmodium falciparum, responsible for most cases of malaria in humans and also for the most severe [cases]. The aim of the study was to find out which factors in the host organism could tell the parasite when to move forward in its life cycle.
“There are many hypotheses to explain the phenomenon, such as the existence of genetic factors or molecules released by the human immune system,” Marti said. “We suspected it might be the host’s nutritional status, meaning that when the parasite notices that the supply of nutrients is limited, it looks for ways of being transmitted to another organism.”
To test his theory, Marti conducted a series of experiments in collaboration with Jon Clardy, a professor of biological chemistry and molecular pharmacology at Harvard Medical School in the US, along with other colleagues.
The scientists found that when conventional blood serum was added to parasite culture, no individuals became gametocytes and all of them continued to reproduce normally in blood cells. However, when the culture was treated with serum that had been previously exposed to parasites, they reproduced more slowly, and some individuals differentiated into gametocytes.
“Something important was missing, so we decided to fractionate the serum and test its components one by one on the cultured parasites,” Marti said.
Initially, the researchers thought blood sugar level was the key factor in the parasite’s phase change, but the experiments showed that more gametocytes formed in the absence of lysophosphatidylcholine, a phospholipid found in abundance in human blood and essential to cell membrane synthesis.
“Our tests showed that this phospholipid is rapidly sequestered by the parasite, which breaks it down and uses its components to synthesize new parasite membranes. In the absence of this nutrient, about 30% of the protozoans that infect blood cells become gametocytes. The other 70% reproduce more slowly,” Marti said.
Blood levels of lysophosphatidylcholine fall by a factor of five during a severe infection, and indeed, this factor is considered a non-specific marker of inflammation. In the case of malaria, the phospholipid appears to act as an environmental sensor for the parasite.
“The parasite develops in a constantly changing environment in both the human host and the mosquito,” Marti said. “It has to sample the environment frequently and adjust its metabolism whenever necessary.”
He added that the same mechanism is probably present in P. vivax, the species that causes most malaria cases in Brazil, but the hypothesis cannot be tested in the laboratory owing to the low viability of this species in culture.
Knowledge gaps
Understanding exactly how the absence of lysophosphatidylcholine results in gametocyte formation is one of the current aims of the group led by Marti, but he noted that several other questions also need to be answered.
“Only some parasites advance in their life cycle when we remove the phospholipid from blood serum. Why not all? What mechanism decides which individuals will become gametocytes and which will continue to reproduce asexually? Can it be a random process, or are there other molecules in the extracellular medium that regulate it? We don’t know the answers right now,” he said.
Another future possibility is an investigation to find out whether the host’s diet affects the level of lysophosphatidylcholine, as well as whether this influences the infectious process or transmission of the parasite. It is also possible to investigate how variations in the level of lysophosphatidylcholine during infection in humans affect the transmission process.
“Two of the enzymes used by the parasite to metabolize this molecule are already known targets for drugs currently in clinical trial. The new discovery points to the possibility of more directed experiments,” Marti said.
Sensors of the microenvironment
According to SPCell organizer Celia Garcia, a professor at FCF-USP, the studies conducted by Marti are a major contribution to knowledge of Plasmodium’s mechanisms for signaling and communication with the host microenvironment during the gametocyte phase.
In her own laboratory, Garcia is running a complementary effort to understand how the parasite perceives the host microenvironment while it is developing inside red blood cells.
“The study of cell signaling in the parasite-host relationship has advanced significantly in recent years. Many papers have been published by several labs, reinforcing the relevance of this idea that the malaria parasite has sensors of the microenvironment to regulate aspects of its life cycle. This idea was unthinkable or at least highly controversial only a few years ago,” Garcia said.
The article “Lysophosphatidylcholine regulates sexual stage differentiation in the human malaria parasite Plasmodium falciparum” by Matthias Marti, Jon Clardy et al. can be read at: cell.com/cell/fulltext/S0092-8674(17)31242-4.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.




